Abstract
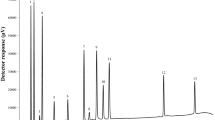
Two chromatographic methods have been described for the simultaneous determination of metronidazole (MET) and spiramycin (SPY) in their mixtures. The first method was based on a high performance thin layer chromatographic (HPTLC) separation of the two drugs followed by densitometric measurements of their spots at 240 nm. The separation was carried out on Merck TLC aluminum sheets of silica gel 60 F254 using methanol: chloroform (9:1, v/v) as a mobile phase. Analysis data was used for the linear regression line in the range of 1.0–2.0 and 0.8–2.0 μg band−1 for MET and SPY, respectively. The second method was based on a reversed-phase liquid chromatographic separation of the cited drugs on a C-18 column (5 μm, 250 × 4.6 mm, i.d.). The mobile phase consisted of a mixture of phosphate buffer of pH 2.4 and acetonitrile (70:30, v/v). The separation was carried out at ambient temperature with a flow rate of 1.0 mL min−1. Quantitation was achieved with UV detection at 232 nm based on peak area with linear calibration curves at concentration ranges 0.4–50.0 and 0.5–50.0 μg mL−1 for MET and SPY, respectively. The proposed chromatographic methods were successfully applied to the determination of the investigated drugs in pharmaceutical preparations. Both methods were validated in compliance with ICH guidelines; in terms of linearity, accuracy, precision, robustness, limits of detection and quantitation and other aspects of analytical validation.


Similar content being viewed by others
References
Hardman JG, Limbird LE (1996) Goodman & Gillman’s, The Pharmacological Basis of Therapeutics, 9th ed. McGraw, Hill, NewYork
Zhong D, Shi X, Sun L, Chen X (2003) J Chromatogr B 791:45–53
British Pharmacopoeia (2008) through internet communications, http://www.pharmacopoeia.co.uk/ixbin/bp.cgi
Sagan C, Salvador A, Dubreuil D, Poulet PP, Duffaut D, Brumpt I (2005) J Pharm Biomed Anal 38:298–306
Li F, Ye YY, Jia WP, Chen Q (2006) Yaowu Fenxi Zazhi 26:1311–1313
Menelaou A, Somogyi AA, Barclay ML, Bochner F (1999) J Chromatogr B 731:261–266
Mishal A, Sober D (2005) J Pharm Biomed Anal 39:819–823
Jin W, Li W, Xu Q, Dong Q (2000) Electrophoresis 21:1409–1414
Agbaba D, Djurkovic M, Brboric J, Zivanov-stakic D (1998) J Planar Chromatogr Mod TLC 11:447–449
Bartlett PN, Ghoneim E, El-Hefnawy G, El-Hallag I (2005) Talanta 66:869–874
Huet AC, Mortier L, Els Daeseleire, Fodey T, Elliott C, Delahaut P (2005) Anal Chim Acta 534:157–162
Liu JF (1999) Yaowu Fenxi Zazhi 19:125–126
Kanfer I, Skinner MF, Walker RB (1998) J Chromatogr A 812:255–286
Horie M, Saito K, Ishii R, Yoshida T, Haramaki Y, Nakazawa H (1998) J Chromatogr A 812:295–302
Garcia-Mayor MA, Garcinuno RM, Fernandez-Hernado P, Durand-Alergia JS (2006) J Chromatogr A 1122:76–83
Flurer CL (1996) Electrophoresis 17:359–366
Sun C, Yu R, Yang Q, Sheng S, Zhao X (1987) Yaoxue Xuebao 22:515–519
Ozkan SA, Uslu B, Aboul-Enein HY (2003) Crit Rev Anal Chem 33:155–181
Moffat AC, Osselton MD, Widdop B (2004) Clarke’s Analysis of Drugs and Poisons 3 rd ed., London, pp. 915–916, 1319–1320
Q2A, ICH, Q2A (R1), Validation of Analytical Procedures: Text and Methodology, International Conference on Harmonization, Geneva, (November 2005), (http://www.ich.org/LOB/media/MEDIA417.pdf)
Kaul N, Dhaneshwar SR, Agrawal H, Kakad A, Patil B (2005) J Pharm Biomed Anal 37:27–38
Miller JN, Miller JC (2000) Statistics and Chemometrics for Analytical Chemistry, 4th ed edn. Prentice Hall, Harlow, England, pp 111–118
Armitage P, Berry G (1994) Statistical Methods in Medical Research, 3rd ed edn. Blackwell Scientific Publications, Oxford, England, pp 283–285
Sethi PD (1996) High Performance Thin Layer Chromatography: Quantitative Analysis of Pharmaceutical Formulations. CBS Publishers and Distributors, New Delhi, India
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maher, H.M., Youssef, R.M. Development of Validated Chromatographic Methods for the Simultaneous Determination of Metronidazole and Spiramycin in Tablets. Chroma 69, 345–350 (2009). https://doi.org/10.1365/s10337-008-0865-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0865-2