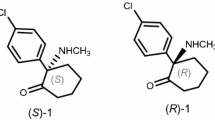
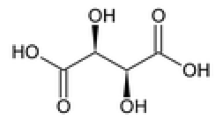
Abstract
Direct resolution of the enantiomers of the racemic drugs ketamine and lisinopril has been achieved by TLC. Enantiomerically pure tartaric acid and mandelic acid were used as chiral impregnating reagents and as mobile phase additives. When (−)-mandelic acid was used as chiral impregnating reagent use of ethyl acetate–methanol–water 3:1:1 (v/v) as mobile phase enabled successful resolution of the enantiomers of both compounds. For lisinopril, the mobile phase acetonitrile–methanol–water–dichloromethane 7:1:1:0.5 (v/v) was successful when (+)-tartaric acid was used as impregnating agent. When (+)-tartaric acid was used as mobile phase additive the mobile phase acetonitrile–methanol–(+)-tartaric acid (0.5% in water, pH 5)–glacial acetic acid 7:1:1.1:0.7 (v/v) enabled successful resolution of the enantiomers of lisinopril. The effects on resolution of temperature, pH, and the amount of chiral selector were also studied. The separated enantiomers were isolated and identified. Spots were detected with iodine vapour. LODs were 0.25 and 0.27 μg for each enantiomer of ketamine with (+)-tartaric acid and (−)-mandelic acid, respectively, whereas for lisinopril LODs were 0.14 and 0.16 μg for each enantiomer with (+)-tartaric acid (both conditions) and (−)-mandelic acid, respectively.


Similar content being viewed by others
References
Bhutta AT (2007) Semin Perinatol 31:303–308. doi:10.1053/j.semperi.2007.07.005
White PF, Ham J, Way WL, Trevor AJ (1980) Anesthesiology 52:231–239. doi:10.1097/00000542-198003000-00008
Ihmsen H, Geisslinger G, Schuttler J (2001) Clin Pharmacol Ther 70:431–438. doi:10.1067/mcp.2001.119722
Oye I, Paulsen O, Maurset A (1992) J Pharmacol Exp Ther 260:1209–1213
Marietta MP, Way WL, Castagnoli N, Trevor AJ (1977) J Pharmacol Exp Ther 202:157–165
White PF, Shuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ (1985) Br J Anaesth 57:197–203. doi:10.1093/bja/57.2.197
White PF, Way WL, Trevor AJ (1982) Anesthesiology 56:119–136
Reich DL, Silvay G (1989) Can J Anaesth 36:186–197
Himmelseher S, Durieux ME (2005) Anesth Analg 101:524–534. doi:10.1213/01.ANE.0000160585.43587.5B
Koinig H, Marhofer P (2003) Paediatr Anaesth 13:185–187. doi:10.1046/j.1460-9592.2003.01000.x
Pees C, Haas NA, Ewert P, Berger F, Lange PE (2003) Pediatr Cardiol 24:424–429. doi:10.1007/s00246-002-0356-4
Mutschler E, Derendorf H, Schhäfer-Korting K, Elord K, Estes S (1995) In: Basic principles and therapeutic aspects. Medpharm Scientific Publishers, Stuttgart, pp 385–390
Oi N, Kitahara H, Matsushita Y, Kisu N (1996) J Chromatogr A 722:229–232. doi:10.1016/0021-9673(95)00665-6
Stringham RW, Ye YK (2006) J Chromatogr A 1101:86–93. doi:10.1016/j.chroma.2005.09.065
Rodriguez RME, Patel S, Wainer IW (2003) J Chromatogr B Analyt Technol Biomed Life Sci 794:99–108. doi:10.1016/S1570-0232(03)00420-3
Svensson JO, Gustafsson LL (1996) J Chromatogr B Analyt Technol Biomed Life Sci 678:373–376. doi:10.1016/0378-4347(95)00545-5
Adams JD Jr, Woolf TF, Trevor AJ, Williams LR, Castagnoli N Jr (1982) J Pharm Sci 71:658–661. doi:10.1002/jps.2600710613
Han X, Berthod A, Wang C, Huang K, Armstrong DW (2007) Chromatographia 65:381–400. doi:10.1365/s10337-007-0182-1
Theurillat R, Knobloch M, Schmitz A, Lassahn P-G, Mevissen M, Thormann W (2007) Electrophoresis 28:2748–2757. doi:10.1002/elps.200600820
Qin XZ, Nguyen DST, Ip DP (1993) J Liq Chromatogr 16:3713–3734. doi:10.1080/10826079308019663
Berger TA, Smith J, Fogelman K, Kruluts K (2002) Am Lab 34:14–20
Bhushan R, Martens J (2003) In: Sherma J, Fried B (eds) Handbook of TLC, 3rd edn. Marcel Dekker, New York, pp 373–415
Bhushan R, Ali I (1987) J Chromatogr A 392:460–463. doi:10.1016/S0021-9673(01)94292-X
Bhushan R, Martens J, Arora M (2001) Biomed Chromatogr 15:151–154. doi:10.1002/bmc.55
Bhushan R, Tanwar S (2008) Biomed Chromatogr 22:1028–1034. doi:10.1002/bmc.1025
Prieto JA, Akesolo U, Jimenez RM, Alonso RM (2001) J Chromatogr A 916:279–288. doi:10.1016/S0021-9673(01)00565-9
Bhushan R, Ali I (1987) Chromatographia 23:141–144. doi:10.1007/BF02312891
Armstrong DW, Chen S, Chang C, Chang S (1992) J Liq Chromatogr 15:545–556. doi:10.1080/10826079208017191
Matchet MW, Branch SK, Jefferies TM (1996) Chirality 8:126–130. doi:10.1002/(SICI)1520-636X(1996)8:1<;126::AID-CHIR18>;3.0.CO;2-P
Simmons BR, Stewart TJ (1994) J Liq Chromatogr 17:2675–2690. doi:10.1080/10826079408013407
Bhushan R, Agarwal C (2008) J Planar Chromatogr 21:129–134. doi:10.1556/JPC.21.2008.2.10
Acknowledgment
The authors are grateful to the Ministry of Human Resources Development, Government of India, New Delhi, for the award of a research assistantship (to C.A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhushan, R., Agarwal, C. Direct TLC Resolution of (±)-Ketamine and (±)-Lisinopril by Use of (+)-Tartaric Acid or (−)-Mandelic Acid as Impregnating Reagents or Mobile Phase Additives. Isolation of the Enantiomers. Chroma 68, 1045–1051 (2008). https://doi.org/10.1365/s10337-008-0856-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0856-3