Abstract
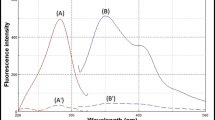
Tramadol was found to exhibit weak fluorescence with a maximum emission at 300 nm when excited at 200 nm. Also, fluorescence spectra of the drug and its two main metabolites, O-desmethyltramadol and N-desmethyltramadol are not practically identical. Thus low and different sensitivities have been reported for the drug and its metabolites in previously published work. In the present method using 9-fluorenylmethyl chloroformate (FMOC-Cl) as labeling agent, equal and magnified fluorescence intensity were obtained for the analytes. The drug, its metabolites and an internal standard (oseltamivir phosphate) were extracted from serum by dichloromethane. Pre-column derivatization of the analytes was achieved using FMOC-Cl in the presence of borate buffer (0.1 M, pH 7.5). Liquid chromatography with a mobile phase consisting of a mixture of 0.05 M phosphate buffer containing triethylamine (2 ml L−1; pH = 3.0) and methanol (54:46; v/v) and a Shimpack CLC-ODS column were used for analytical separation of the analytes. The fluorescence of the column effluent was monitored at an excitation and emission wavelengths of 265 and 315 nm, respectively. The analytical method was linear over the concentration range of 1.0–1,280 ng mL−1 of the parent drug and its metabolites and limit of quantification of 1.0 ng mL−1 was obtained for the analytes using 10 μL injection. The method validation was studied and the validated method applied in a bioequivalence study of 2 different tramadol preparations in 24 healthy volunteers.



Similar content being viewed by others
References
Lee CR, McTavis D, Sorkin E (1993) Drugs 46:313–340. doi:10.2165/00003495-199346020-00008
Tao Q, Stone DJ Jr, Borenstein MR, Jean-Bart V, Codd EE, Coogan TP, Desai-Krieger D, Liao S, Raffa RB (2001) J Chromatogr B 763:165–171. doi:10.1016/S0378-4347(01)00388-7
Ho S-T, Wang J-J, Liaw W-J, Ho Ch-M, Li J-H (1999) J Chromatogr B 736:89–96. doi:10.1016/S0378-4347(99)00434-X
Sha YF, Shenb S, Duan GL (2005) J Pharm Biomed Anal 37:143–147. doi:10.1016/j.jpba.2004.09.050
Gambaro V, Benvenuti C, Ferrari LD, Acqua LD, Fare F (2003) II Farmaco 58:947–950. doi:10.1016/S0014-827X(03)00153-8
Valle M, Pavon JM, Calvo R, Campanero MA, Troconiz IF (1999) J Chromatogr B 724:83–89. doi:10.1016/S0378-4347(98)00547-7
Kmetec V, Roskar R (2003) J Pharm Biomed Anal 32:1061–1066. doi:10.1016/S0731-7085(03)00209-7
Gan SH, Ismail R, Wan Adnan WA, Wan Z (2002) J Chromatogr B 772:123–129. doi:10.1016/S1570-0232(02)00065-X
Yeh GC, Sheu MT, Yen CL, Wang YW, Liu CH, Ho HO (1999) J Chromatogr B 723:247–253
Elsing B, Blaschke G (1999) J Chromatogr 612 (2) (1993) 223–30
Overbeck P, BlaschkeJ G (1999) Chromatogr B Biomed Sci Appl 732(1):185–192
Gan SH, Ismail R (2001) J Chromatogr B 759:325–335. doi:10.1016/S0378-4347(01)00237-7
K¨uc¨uka A, Kadıoglub Y, Celebi F (2005) J Chromatogr B 816:203–208. doi:10.1016/j.jchromb.2004.11.031
Nobilis M, Kopecky J, Kvetina J, Chladek J, Svoboda Z, Vorısek V, Perlık F, Pour M, Kunes J (2002) J Chromatogr A 949:11–22. doi:10.1016/S0021-9673(01)01567-9
Gu Y, Fawcett JP (2005) J Chromatogr B 821:240–243. doi:10.1016/j.jchromb.2005.05.003
Rouini MR, Hosseinzadeh Ardakani Y, Soltani F, Aboul-Enein HY, Foroumad A (2006) J Chromatogr B 830:207–211. doi:10.1016/j.jchromb.2005.10.039
Soetebeer UB, Schierenberg M-O, Moller J-G, Schulz H, Grunefeld G, Andresen P, Blaschke G, Ahr G (2000) J Chromatogr A 895:147–155. doi:10.1016/S0021-9673(00)00704-4
Acknowledgments
This work was supported by Bakhtar Bioshimi Pharmaceutical Company and partially by Kermanshah University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bahrami, G., Mohammadi, B. Enhancement of Fluorescence Intensity of Tramadol and Its Main Metabolites in LC Using Pre-Column Derivatization with 9-Fluorenylmethyl Chloroformate. Chroma 68, 935–940 (2008). https://doi.org/10.1365/s10337-008-0806-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0806-0