Abstract
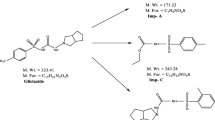
A simple, sensitive gradient RP-LC assay method has been developed for the quantitative determination of amtolmetin guacyl in bulk drug, used as anti-inflammatory drug. The developed method is also applicable for related substances determination. Efficient chromatographic separation was achieved on a C18 stationary phase with simple mobile phase combination delivered in a gradient mode and quantification was carried out using ultraviolet detection at 313 nm at a flow rate of 1.0 mL min−1. In the developed LC method the resolution between Amtolmetin Guacyl and its three potential impurities was found to be greater than 2.0. Regression analysis shows an r value (correlation coefficient) greater than 0.99 for amtolmetin guacyl and its three impurities. This method was capable to detect all three impurities of amtolmetin guacyl at a level of 0.002% with respect to test concentration of 0.5 mg mL−1 for a 10 μL injection volume. The inter- and intra-day precision values for all three impurities and for amtolmetin guacyl was found to be within 2.0% RSD at its specification level. The method has shown good and consistent recoveries for amtolmetin guacyl (99.2–101.5%) and its three impurities (94.5–104.8%). The test solution was found to be stable in diluent for 48 h. The drug was subjected to stress conditions of hydrolysis, oxidation, photolysis and thermal degradation. Considerable degradation was found to occur in oxidative stress conditions. The stress samples were assayed against a qualified reference standard and the mass balance was found close to 99.6%. The developed RP-LC method was validated with respect to linearity, accuracy, precision and robustness.








Similar content being viewed by others
References
Tubaro E, Belogi L, MezzadriM (2000) Eur J Pharmacol 387:233–244. doi:10.1016/S0014-2999(99)00791-8
Allinger N (1977) J Am Chem Soc 99:8127–8134. doi:10.1021/ja00467a001
Burkert U, Allinger NL (1982) In: Molecular mechanics, vol 99. American Chemical Society, Washington, DC, pp 8127–8134
Tubaro E, Belogi L, Mezzadri CM, Ruco L, Stoppacciaro A (1995) Arzneim-Forsch 45:1298–1302
Lo GP, Buffone G, Careddu A, Magni G, Quagliata T, Pacifici L, Carminati P (1998) National meeting of digestive diseases, Milan, November 14–18
Bianchi PG, Monitrone F, Lazzaroni M, Manzionna G, Caruso I (1999) J Gastroenterol Hepatol 31:378–385
Stability ICH Testing of New Drug Substances and Products Q1A (R2) (2003) International conference on Harmonization, IFPMA, Geneva
Singh S, Bakshi M (2000) Pharm Tech On-line 24:1–14
ICH Photo stability Testing of New Drug Substances and Products Q1B (1996) International Conference on Harmonization, IFPMA, Geneva
Bakshi M, Singh S (2002) J Pharm Biomed Anal 28:1011–1040. doi:10.1016/S0731-7085(02)00047-X
Singh S, Singh B, Bahuguna R, Wadhwa L, Saxena R (2006) J Pharm Biomed Anal 41:1037–1040. doi:10.1016/j.jpba.2006.01.030
Carstensen JT, Rhodes CT (eds) (2000) Drug stability principles and practices, 3rd edn, Informa Healthcare, London
Draft ICH Guidelines on Validation of Analytical Procedures (1995) Definitions and Terminology, Federal Register, vol 60, IFPMA, Switzerland, 11260
Validation of compendial methods (2007) The United States Pharmacopeia, 30th edn, USP30
Swartz ME, Krull IS (1998) Pharm Technol 20:104–109
Acknowledgment
The authors wish to thank the management of United States Pharmacopeia laboratory- India for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subba Rao, D.V., Radhakrishnanand, P., Surendranath, K.V. et al. A Stability Indicating LC Method for Amtolmetin Guacyl. Chroma 68, 567–577 (2008). https://doi.org/10.1365/s10337-008-0743-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0743-y