Abstract
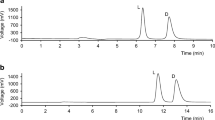
LC separation of carboprost diastereomers in bulk drug was developed and validated using normal-phase amylose stationary phase Chiralpak AD-H. The effect of the organic modifiers, namely 2-propanol and ethanol in the mobile phase was optimized in order to obtain the best separation. The retention time of (R)-carboprost and (S)-carboprost were 15.3 and 17.1 min, respectively. Calibration curves were linear over the range of 0.2–1.0%, with the regression coefficient (R 2) of 0.9997. The limit of detection (LOD) and the limit of quantification (LOQ) were 0.07 and 0.2%, respectively. The method was accurate, precise and suitable to use for the purpose of controlling unwanted (R)-isomer in the carboprost active pharmaceutical ingredient. This method can be successfully applied to the analysis of chiral purity of carboprost in pharmaceutical bulk drug samples.






Similar content being viewed by others
References
Hiriyanna SG, Basavaiah K, Pati HN, Mishra BK (2007) J Liq Chromatogr & Rel Tech 30:3093–3105
Ariens EJ (1984) Eur J Clin Pharmacol 26:663–668. doi:10.1007/BF00541922
Ariens EJ (1986) Med Res Rev 6:451–456. doi:10.1002/med.2610060404
Ariens EJ, Wuins EW (1987) Clin Pharmacol Ther 42:361–363
FDA policy statement for the development of new stereoisomeric drugs, Washington, DC, May 1992
Muzaffer K, Balaji V, Sreenivas Rao D, Reddy R (2007) J Chromatogr B Anal Technol Biomed Life Sci 846:119–123. doi:10.1016/j.jchromb.2006.08.033
Silva IJD Jr, Sartor JP, Rosa PCP, Veredas VD, Barreto AJ, Santana CC (2007) J Chromatogr A 1162:97–102. doi:10.1016/j.chroma.2007.07.008
Chu KO, Wang CC, Pang CP, Rogers MS (2007) J Chromatogr B Anal Technol Biomed Life Sci 857:83–91. doi:10.1016/j.jchromb.2007.07.016
Bygdeman M (2003) Best Pract Res 17:707–716
Yankee EW, Axen U, Bundy GL (1974) J Am Chem Soc 96:5865–5876. doi:10.1021/ja00825a027
Robert A, Yankee EW (1975) Proc Soc Exp Biol Med 148:1155–1158
Hamberg M, Zhang LY, Bergstrom S (1995) Eur J Pharm Sci 3:27–38. doi:10.1016/0928-0987(94)00072-8
Willams MS, Tsibris JCM, Davis G, Baiano J, Brien WFO (2002) Am J Obstet Gynecol 187:615–619. doi:10.1067/mob.2002.124959
Womack IM, Lee AS, Kamath B, Agrawal KC, Kishore V (1996) Prostaglandins 52:249–259. doi:10.1016/S0090-6980(96)00087-1
Watzer B, Seyberth HW, Schweer H (2002) J Mass spectrum 37:927–933
USP 31 (2008) 2:1646
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiriyanna, S.G., Basavaiah, K., Dhayanithi, V. et al. Chiral Separation of Carboprost Isomers by Normal Phase LC Using Amylose Chiral Stationary Phase. Chroma 68, 501–505 (2008). https://doi.org/10.1365/s10337-008-0742-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0742-z