Abstract
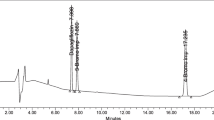
A GC–MS method for the simultaneous determination of two process related toxic impurities viz. 2-(chloromethyl)-3,4-dimethoxypyridine hydrochloride (CDP) and dimethyl sulfate (DMS) and RP-LC for the routine determination of CDP in pantoprazole sodium (PPS) are presented. In GC–MS, a temperature gradient program was performed on a capillary DB-624 column (60 m × 0.32 mm × 1.8 μm). LC analysis of CDP was done on a Novaflex C18 (250 × 4.6 mm, 5 μm) column using mobile phase containing buffer (0.02 M potassium dihydrogen phosphate and 0.0025 M di potassium hydrogen phosphate) and acetonitrile in 46:54 v/v ratio. The flow rate was 1.0 mL min−1 and the elution was monitored at 220 nm. Both methods were validated as per International Conference on Harmonization (ICH) guidelines. GC–MS is able to quantitate up to 3.0 ppm of CDP and DMS whereas with RP-LC up to 9.0 ppm of CDP could be quantitated.


Similar content being viewed by others
References
The Merck Index (Merck & Co., Inc. Whitehouse Station, NJ, USA) (2006) 14th edn. pp 1209
Elder DP, Teasdale A, Lipczynski AM (2008) J Pharm Biomed Anal 46:1
European medicines agency, guideline on the limits of genotoxic impurities, CPMP/SWP/5199/02, EMEA/CHMP/QWP/251344/2006 (2007)
Thomson PDR, Montvale NJ (2007) Physicians desk reference. 61:3469–3475
Rajic KK, Novovic D, Marinkovic V, Agbaba D (2003) J Pharm Biomed Anal 32:1019
El-Sherif ZA, Mohamed AO, El-Bardicy MG, El-Tarras MF (2006) Chem Pharm Bull 54(6):814
Reddy GM, Vijaya Bhaskar B, Pratap Reddy P, Ashok S, Sudhakar P, Moses Babu J, Vyas K, Mukkanti K (2007) J Pharm Biomed Anal 45:201
Sivakumar T, Rajappan M, Kannappan V (2008) Chromatographia 67:41
International Conference on Harmonisation Guideline on Validation of Analytical Procedures; Q2 (R1); (2005)
Acknowledgments
The authors are highly thankful to Dr. Bandi Parthasarathi Reddy, CMD, Hetero Drugs Limited, Hyderabad, India for his encouragement and providing facilities to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raman, N.V.V.S.S., Reddy, K.R., Prasad, A.V.S.S. et al. Validated Chromatographic Methods for the Determination of Process Related Toxic Impurities in Pantoprazole Sodium. Chroma 68, 481–484 (2008). https://doi.org/10.1365/s10337-008-0718-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0718-z