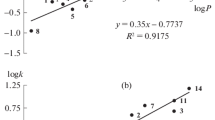
Abstract
In order to elucidate the influence of the mobile phase composition on the retention of some 1-aralkyl-4-arylpiperazines, Snyder’s selectivity and polarity concept for building a simple linear solvation energy relationships model of chromatographic behavior was used. Maximum variability of mobile phase selectivity was achieved by using ternary mixtures of solvents from three different corners of the Snyder selectivity triangle: methanol as a strong proton donor, acetone as a strong proton acceptor and dimethyl formamide as a dipole interactor. Water was added to keep elution strength constant. Mobile phase composition was varied using Simplex–lattice mixture design. Selectivity parameters were calculated on the basis of linear relationships between volume fractions of pure solvents and their χ e , χ d , χ n values. For seven-substituted 1-aralkyl-4-arylpiperazines R M values were measured and correlated with previously calculated selectivity parameters. Mathematical models obtained are discussed according to the structural properties of the studied compounds.
Similar content being viewed by others
References
Kamlet MJ, Abboud JL, Taft RW (1977) J Am Chem Soc 99:6027–6038. doi:10.1021/ja00460a031
Taft RW, Kamlet MJ (1976) J Am Chem Soc 98:2886–2894. doi:10.1021/ja00426a036
Taft RW, Kamlet MJ (1976) J Am Chem Soc 98:377–383. doi:10.1021/ja00426a036
Kamlet MJ, Abboud JL, Abraham MH, Taft RW (1983) J Org Chem 48:2877–2887. doi:10.1021/jo00165a018
Sadek PC, Carr PW, Doherty RM, Kamlet MJ, Taft RW, Abraham MH (1985) Anal Chem 57:2971–2978. doi:10.1021/ac00291a049
Carr PW, Doherty RM, Kamlet MJ, Taft RW, Melander W, Horvath C (1986) Anal Chem 58:2674–2680. doi:10.1021/ac00126a021
Carr PW (1993) Microchem J 48:4–28. doi:10.1006/mchj.1993.1066
Carr PW (1998) J Chromatogr A 799:1–19. doi:10.1016/S0021-9673(97)01054-6
Tan LC, Carr PW, Abraham M (1996) J Chromatogr A 752:1–18. doi:10.1016/S0021-9673(96)00459-1
Abraham MH (1993) Chem Soc Rev 22:73–83. doi:10.1039/cs9932200073
Abraham MH, Roses M (1994) J Phys Org Chem 7:672–684. doi:10.1002/poc.610071205
Abraham MH, Roses M, Poole CF, Poole SK (1997) J Phys Org Chem 10:358–368. doi :10.1002/(SICI)1099-1395(199705)10:5<358::AID-POC907>3.0.CO;2-N
Roses M, Boillet P, Poole CF (1998) J Chromatogr A 829:29–40. doi:10.1016/S0021-9673(98)00746-8
Boillet P, Poole CF, Roses M (1998) Anal Chim Acta 368:129–140. doi:10.1016/S0003-2670(98)00190-1
Jones JL, Rutan SC (1991) Anal Chem 63:1318–1322. doi:10.1021/ac00013a026
Lu H, Rutan SC (1996) Anal Chem 68:1387–1393. doi:10.1021/ac9507810
Helburn RS, Rutan SC, Pompano J, Mitchem D, Patterson WT (1994) Anal Chem 66:610–618. doi:10.1021/ac00077a006
Snyder LR (1974) J Chromatogr A 92:223–230. doi:10.1016/S0021-9673(00)85732-5
Rutan SC, Carr PW, Cheong WJ, Park JH, Snyder LR (1989) J Chromatogr A 463:21–37. doi:10.1016/S0021-9673(01)84451-4
Zhang X, Hodgetts K, Rachwal S, Zhao H, Wasley J, Craven K et al (2000) J Med Chem 43:3923–3932. doi:10.1021/jm990562x
Andrić D, Tovilović G, Roglić G, Šoškić V, Tomić M, Kostić-Rajačić S (2007) J Serb Chem Soc 72:747–755. doi:10.2298/JSC0709747A
Šukalović V, Andrić D, Roglić G, Kostić-Rajačić S, Schrattenholz A, Šoškić V (2005) Eur J Med Chem 40:481–493. doi:10.1016/j.ejmech.2004.10.006
Veličković JĐ, Andrić D, Roglić G, Tešić ŽL, Milojković-Opsenica DM (2004) J Planar Chromatogr 17:255–260. doi:10.1556/JPC.17.2004.4.3
Veličković JĐ, Tešić ŽL, Milojković-Opsenica DM (2005) J Planar Chromatogr 18:358–364. doi:10.1556/JPC.18.2005.5.4
Johnson BP, Khaledi MG, Dorsey JG (1986) Anal Chem 58:2354–2365. doi:10.1021/ac00125a003
Nyiredy S (2004) J Chromatogr B Analyt Technol Biomed Life Sci 812:35–51
Aaranjo P (2000) Trends Analyt Chem 19:524–529. doi:10.1016/S0165-9936(00)00035-2
Gennaro MC, Marengo E, Gianotti V, Angioni S (2002) J Chromatogr A 945:287–292. doi:10.1016/S0021-9673(01)01487-X
Chen J, Glancy K, Chen X, Alesandro M (2001) J Chromatogr A 917:63–73. doi:10.1016/S0021-9673(01)00670-7
Ye C, Liu J, Ren F, Okafo N (2000) J Pharm Biomed Anal 23:169–174. doi:10.1016/S0731-7085(00)00335-6
Ficcara R, Ficcara P, Tommasini S, Melardi S, Calabro ML, Furlanetto S et al (2000) J Pharm Biomed Anal 23:169–174. doi:10.1016/S0731-7085(00)00266-1
Ficcara R, Calabro ML, Cutroneo P, Tommasini S, Melardi S, Semereen M et al (2002) J Pharm Biomed Anal 29:1097–1103. doi:10.1016/S0731-7085(02)00151-6
Ragonese R, Mulholand M, Kalman J (2000) J Chromatogr A 870:45–51. doi:10.1016/S0021-9673(99)00972-3
Fabre H (1996) J Pharm Biomed Anal 14:1125–1132. doi:10.1016/S0731-7085(96)01770-0
Ferreiros N, Iriarte G, Alonso RM, Jimenez RM (2007) Talanta 73:748–756. doi:10.1016/j.talanta.2007.04.062
Boillet D, Poole CF (1998) Anal Commun 35:253–256. doi:10.1039/a803247e
Brereton RG (2003) In: Chemometrics, data analysis for the laboratory and chemical plant. Wiley, Chichester
Acknowledgments
This work has been supported by the Ministry of Science of Serbia, Grant 142062.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrić, F.L., Trifković, J.Đ., Tešić, Ž.L. et al. An Approximate Linear Solvation Energy Relationships Model Based on Snyder’s Selectivity Parameters. Chromatographic Behavior of Some 1-Aralkyl-4-Arylpiperazines. Chroma 68, 453–458 (2008). https://doi.org/10.1365/s10337-008-0711-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0711-6