Abstract
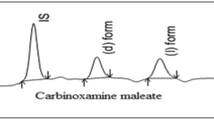
A high-performance liquid chromatographic method has been developed for resolution of the structural isomers of fexofenadine hydrochloride in the bulk drug. The isomers were resolved to baseline on a reversed-phase ODS column with pH 3 aqueous buffer–acetonitrile 60:40 containing 5 g L−1 β-cyclodextrin as mobile phase additive. The aqueous buffer was prepared by dissolving 6.8 g KH2PO4 in 1,000 mL water and adjusting to the pH 3.0 with orthophosphoric acid. Resolution for the structural isomers was not less than 3.0. The method was extensively validated and proved to be accurate and precise. The calibration plot was indicative of an excellent linear relationship between response and concentration over the range 0.025–3.750 ppm for the meta isomer. The limits of detection and quantification for the meta isomer were 0.003 and 0.009 ppm, respectively, for an injection volume of 20 μL. Recovery of the meta isomer from bulk drug samples of fexofenadine HCl was 98.43, 96.52, and 97.72% for addition of 0.5, 0.6, and 0.7%, respectively. The analytical solution was stable for 48 h. The method was found to be accurate, and suitable for quantitative determination of the meta isomer in the bulk drug; it can also be used as a stability-indicating method for assay of fexofenadine.


Similar content being viewed by others
References
Nelson HS, Reynold R, Mason J (2000) Ann Allergy Asthma Immunol 84(5):517–522
Mason J, Reynold R, Rao N (1999) Clin Exp Allergy 29(s3):163–170
Katagiri K, Arakawa S, Hatano Y, Fujiwara S (2006) J Dermatol 33(2):75–79
Baena-Cagnani CE, Finn A, Potter P, Meltzer EO, Wahn U, Emeryk A, Hardy P, Ruuth E (2006) J World Allergy 18(5):184–191
Sahajwalla S (2004) In: Sahajwalla CG (ed) New drug development. Marcel Dekker, New York, pp 421–426
Arai T, Koike H, Hirata K, Oizumi H (1988) J Chromatogr A 448:439–444
Zeng S, Zhong J, Pan L, Li Y (1999) J Chromatogr B 728:151–155
Wong FA, Juzwin SJ, Flor SC (1997) J Pharm Biomed Anal 15(6):765–771
International Conference on Harmonization (1996) International conference on harmonization tripartite guideline Q2B, ICH Secretariat, Geneva, November
Coutrim MX, Nakamura LA, Collins CH (1993) Chromatographia 37:185–190
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakalgaonkar, A.A., Mirgane, S.R. & Pawar, R.P. Validated LC Method, with a Chiral Mobile Phase, for Separation of the Isomers of Fexofenadine Hydrochloride. Chroma 68, 143–146 (2008). https://doi.org/10.1365/s10337-008-0667-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0667-6