Abstract
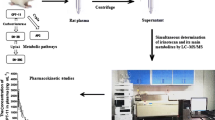
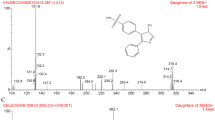
SHR110008 is a representative 9-β-dihydro-9,10-O-acetal taxane with greater anticancer activity and less toxicity than docetaxel. To support a preclinical study of its pharmacokinetics and to predict the effect of 9-β-dihydro-9,10-O-acetal modification on its pharmacokinetic properties, we have developed a sensitive and rapid liquid chromatographic–tandem mass spectrometric method for quantitative analysis of SHR110008 in rat and dog plasma. Plasma was extracted with ethyl acetate. The analytes were separated on a 150 × 4.6 mm i.d., 5 μm particle, reversed-phase C18 column with 90:10 (v/v) methanol–0.1% formic acid as mobile phase at a flow rate of 0.3 mL min−1. Detection was performed by triple-quadrupole tandem mass spectrometry in selected reaction monitoring (SRM) mode with an electrospray ionization source. The precursor-to-product ion transition m/z 933 → 142 was used. The method was validated for accuracy and precision, and linearity in the two matrices was good. Lower limits of quantification (LLOQ) in rat and dog plasma were 5 and 2 ng mL−1, respectively. There were no stability-related problems in the procedure for analysis of SHR110008. The method was successfully used in a preclinical study of the pharmacokinetics of SHR110008 in rats and beagle dogs. The pharmacokinetics of SHR110008 were non-linear in rats and dogs. The elimination half-life ranged from 5.18 to 7.32 h for the rats and from 6.42 to 8.42 h for the dogs.




Similar content being viewed by others
References
Vaishampayan U, Parchment RE, Jasti BR, Hussain M (1999) Urology 54(6A Suppl):22–29
Schrijvers D, Wanders J, Dirix L, Prove A, Vonck I, Van Oosterom A, Kave S (1993) Ann Oncol 4(7):610–611
Brooks TA, Minderman H, O’Loughlin KL, Pera P, Ojima I, Baer MR, Bernacki RJ (2003) Mol Cancer Ther 2:1195–1205
Nobili S, Landini I, Giglioni B, Mini E (2006) Curr Drug Targets 7:861–879
Van Oosterom AT, Schrijvers D (1995) Anticancer Drugs 6(3):356–368
Takashi I, Shin I, Satoru O, Kouichi U, Hirofumi T, Tsunehiko S (2002) Bioorg Med Chem Lett 12:1083–1086
Yasuyuki T, Toshiharu Y, Kouichi U, Jun C, Takashi I, Michio I, Takeshi J, Noriko T, Hirofumi T, Tsunehiko S (2003) Bioorg Med Chem Lett 13:185–190
Sun P, Lei X, Yuan K (2007) PCT Int Appl, WO 2007079666 A1, p 38
Guo P, Ma J, Li S, Gallo JM (2003) J Chromatogr B 798(1):79–86
Song L, Prey JD, Xue J, Kanter P, Manzotti C, Bombardelli E, Morazzoni P, Pendyala L (2005) Rapid Commun Mass Spectrom 19:3617–3625
Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ (2003) J Chromatogr B 783(1):231–236
Sottani C, Colombo T, Zucchetti M, Fruscio R, D’Incalci M, Minoia C (2001) Rapid Commun Mass Spectrom 15:1807–1816
Guitton J, Cohen S, Tranchand B, Vignal B, Droz J-P, Guillaumont M, Manchon M, Freyer G (2005) Rapid Commun Mass Spectrom 19:2419–2426
Vainchtein LD, Thijssen B, Stokvis E, Rosing H, Schellens JH, Beijnen JH (2006) Biomed Chromatogr 20:139–148
Tong X, Zhou J, TanY (2006) J Chromatogr Sci 44:266–271
Wang LZ, Goh BC, Grigg ME, Lee SC, Khoo YM, Lee HS (2003) Rapid Commun Mass Spectrom 17:1548–1552
Kuppens IE, van Maanen MJ, Rosing H, Schellens JH, Beijnen JH (2005) Biomed Chromatogr 19:355–361
Sottani C, Minoia C, D’Incalci M, Paganini M, Zucchetti M (1998) Rapid Commun Mass Spectrom 12:251–255
US Department of Health and Human Services (2001) Guidance for industry, bioanalytical method validation. Food and Drug Administration, Center for Drug Evaluation and Research
Mortier KA, Zhang GF, Van Peteghem CH, Lambert WE (2004) J Am Soc Mass Spectrom 15:585–592
Bissery MC, Nohynek G, Sanderink GJ, Lavelle F (1995) Anticancer Drugs 6(3):339–355
Monsarrat B, Royer I, Wright M, Cresteil T (1997) Bull Cancer 84:125–133
Acknowledgments
We gratefully thank Professor Sun Fenzhi and Dr. Hao Haiping for kind assistance in the revision of the paper. This research was supported by the National High Technology Foundation of China (‘863’ Project, No. 2003AA2Z347A, 2005AA2Z3C70).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1365/s10337-008-0680-9
Rights and permissions
About this article
Cite this article
Hu, X., Sun, J., Wang, G. et al. LC–MS–MS Study of the Pharmacokinetics of a 9-β-Dihydro-9,10-O-acetal Derivative of Docetaxel in Rats and Beagle Dogs. Chroma 67, 883–892 (2008). https://doi.org/10.1365/s10337-008-0638-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0638-y