Abstract
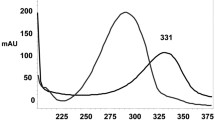
A rapid, selective and sensitive reversed-phase liquid chromatographic (LC) method was developed for the determination of piribedil in human serum, urine and pharmaceutical dosage form. LC analysis was carried out using reversed-phase isocratic elution with a C18 column and a mobile phase of 0.01 M phosphate buffer-acetonitrile (50:50, v/v). The chromatograms showed good resolution and sensitivity with no interference of human serum and urine. Piribedil concentrations were determined using diode array detection at 240 nm. Sildenafil citrate was used as internal standard. The limit of quantification (LOQ) and limit of detection (LOD) concentrations were 107.2 and 321.6 pg mL−1, 96.6 and 290.4 pg mL−1, 161.7 and 53.9 pg mL−1 for urine, serum and pharmaceutical dosage forms, respectively. The method was validated for its linearity, precision and accuracy and applied to the tablets, urine and human serum. In addition, the results were compared to those obtained from UV-spectrophotometry.


Similar content being viewed by others
References
Schuck S, Bentue-Ferrer D, Kleinermans D, Reymann JM, Polard E, Gandon JM, Allain H (2002) Fundam Clin Pharmacol 16:57–65
Janner P, Taylor AR, Campbell DB (1973) J Pharm Pharmacol 25:749–750
Fanelli R, Frigerio A (1974) J Chromatogr A 93:441–446
Abdel-Gawad FM (1997) J Pharm Biomed Anal 15:1679–1685
Regnier GL, Canevari RJ, Laubie MJ, Le Douarec JC (1968) J Med Chem 11:1151–1155
Abdel-Gawad FM (1998) J Pharm Biomed Anal 16:793–799
Abou-Attia FM, Issa YM, Abdel-Gawad FM, Abdel-Hamid SM (2003) Farmaco 58:573–579
Sarati S, Guiso G, Spinelli R, Caccia S (1991) J Chromatogr 563:323–332
Sarati S, Caccia S (1992) Eur J Drug Metab Pharmacokinet 17:205–211
Issa YM, Hassouna MM, Abdel-Gawad FM, Hussien EM (2000) J Pharm Biomed Anal 23:493–502
Yardimci C, Suslu I, Ozaltin N (2004) Anal Bioanal Chem 379:308–311
Ibrahim H (2005) J Pharm Biomed Anal 38:624–632
Uslu B, Ozkan SA (2003) J Pharm Biomed Anal 10:481–489
Sabry SM, Barary MH, Abdel-Hay H, Belal TS (2004) J Pharm Biomed Anal 34:509–516
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altiokka, G., Can, N.Ö. & Aboul-Enein, H.Y. Determination of Piribedil in Human Serum, Urine and Pharmaceutical Dosage Form by LC-DAD. Chroma 67, 905–910 (2008). https://doi.org/10.1365/s10337-008-0626-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0626-2