Abstract
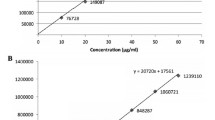
A new, simple high-performance thin-layer chromatographic method has been established and validated for simultaneous determination of escitalopram oxalate and clonazepam in a combined tablet dosage form. The drugs were separated on aluminum plates precoated with silica gel 60 F254; toluene–ethyl acetate–triethylamine 7:3.5:3 (v/v) was used as mobile phase. Quantitative analysis was performed by densitometric scanning at 258 nm. The method was validated for linearity, accuracy, precision, and robustness. The calibration plot was linear over the ranges 250–2,500 and 50–500 ng band−1 for escitalopram oxalate and clonazepam, respectively. The method was successfully applied to the analysis of drugs in a pharmaceutical formulation.


Similar content being viewed by others
References
Escitalopram oxalate. RxList Website. http://www.rxlist.com/cgi/generic/lexapro.htm
Garriock HA, Delgado P, Kling MA (2006) Behav Brain Funct 2:24
United States Pharmacopeia (2006) The United States Pharmacopeia official compendia of standards, 29th edn. USP Convention, Rockville, MD, pp 555–556
Parker WA (1988) Epilepsy. In: Herfindal ET, Gourley DR, Hart LL (eds) Clinical pharmacy and therapeutics. Williams and Wilkins, Maryland, pp 585–586
Singh SS, Shah H, Gupta S (2004) J Chromatogr B 811:209–215
Greiner C, Hiemke C, Bader W, Haen E (2007) J Chromatogr B 848:391-394
Kosel M, Eap CB, Amey M, Baumann P (1998) J Chromatogr B 719:234–238
Nevado JJB, Cabanillas CG, Llerena MJV, Robledo VR (2005) J Chromatogr A 1072:249–257
Bares IF, Pehourcq F, Jarry C (2004) J Pharm Biomed Anal 36:865–869
Valenza T, Rosselli P (1987) J Chromatogr 386:363–366
Mahjoub AE, Staub C (2000) J Chromatogr B 742:381–390
Robertson MD, Drummer OH (1995) J Chromatogr B 667:179–184
Bugey A, Staub C (2004) J Pharm Biomed Anal 35:555–562
Mullett WM, Pawliszyn J (2001) J Pharm Biomed Anal 26:899–908
Mahjoub AE, Staub C (2000) J Pharm Biomed Anal 23:447–458
Kabra PM, Nzekwe EU (1985) J Chromatogr B 341:383–390
Randez-Gil F, Daros JA, Salvador A, Guardia MDL (1991) J Pharm Biomed Anal 9:539–545
Salem AA, Barsoum BN, Izake EL (2004) Spectrochim Acta A 60:771–780
Song D, Zhang S, Kohlhof K (1996) J Chromatogr B 686:199–204
Mahjoub AE, Staub C (2000) J Pharm Biomed Anal 23:1057–1063
Spell JC, Stewart JT (1998) J Pharm Biomed Anal 18:453–460
Salem AA, Barsoum BN, Izake EL (2003) Anal Chim. Acta 498:79–91
Neville GA, Beckstead HD, Shurvell HF (1991) Vib Spectrosc 1:287–297
Gandhi SV, Dhavale ND, Jadhav VY, Sabnis SS (2008) JAOAC Int (in press)
International Conference on Harmonization (2005) ICH harmonised tripartite guideline (Nov 2005), Validation of analytical procedures: text and methodology Q2 (R1). ICH, Geneva
Acknowledgments
The authors wish to express their gratitude to Cipla Ltd, Kurkumbh, India, for the sample of pure escitalopram oxalate, and to Torrent Pharmaceutical Ltd, Indrad, Gujarat, India, for the sample of pure clonazepam.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhavale, N., Gandhi, S., Sabnis, S. et al. Simultaneous HPTLC Determination of Escitalopram Oxalate and Clonazepam in Combined Tablets. Chroma 67, 487–490 (2008). https://doi.org/10.1365/s10337-008-0524-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0524-7