Abstract
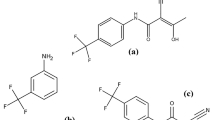
A stability-indicating HPLC method for the quantitative determination of Bicalutamide is described. Bicalutamide is a nonsteroidal antiandrogen and is an oral medication that is used for treating prostate cancer. Separation was achieved on a Waters Symmetry shield RP18 HPLC column using a mobile phase consists of a mixture of phosphate buffer (Solvent A) and organic modifier acetonitrile (Solvent B). Degradation studies were performed on bulk samples of bicalutamide using acid (0.5 N methanolic hydrochloric acid), base (0.5 N methanolic sodium hydroxide), oxidation (10% v/v methanolic hydrogen peroxide), heat (60 °C) and UV light (254 nm). Degradation was observed under base hydrolysis to give the starting material used during the synthesis of bicalutamide. The degraded samples were assayed and gave a mass balance greater than 99.5%, thus proving the stability-indicating power of the developed method. The method was validated with respect to linearity, accuracy, precision and robustness.


Similar content being viewed by others
References
The electronic Medicines Compendium website http://www.medicines.org.uk (January 2006)
Nageswara Rao R, Narasa Raju A, Nagaraju D (2006) J Pharm Biomed Anal 42(3):347
Sweetman et al. (2002) Martindale: the complete drug reference, 33rd edn. Pharmaceutical Press
British National Formulary (50th edition). British Medical Association and Royal Pharmaceutical Society of Great Britain, September (2005)
Roland T, Ádám B, György O, Ferenc L, Daniel W, Antal P (2005) J Chromatogr A 1098:75– 81
Miller JM, Jonathan BC (2000), Analytical chemistry in a GMP environment p 436
International conference on harmonization October (1994), Text on Validation of Analytical Procedures Q2A
International conference on harmonization November (1996) Validation of Analytical Procedures Methodology Q2B
Acknowledgments
The authors wish to thank the management of Dr. Reddy’s group for supporting this work. The authors wish to acknowledge the Process Research Group for providing the samples. We would also like to thank colleagues in the Separation Science Division of Analytical Research of High Potent Active Pharmaceutical Ingredients for their co-operation in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saravanan, G., Rao, B.M., Ravikumar, M. et al. A Stability-Indicating LC Assay Method for Bicalutamide. Chroma 66, 219–222 (2007). https://doi.org/10.1365/s10337-007-0280-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-007-0280-0