Abstract
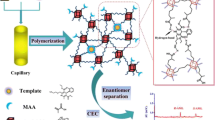
The aim of this study was to observe the chiral separation of a series of C2-asymmetric bi-naphthyl compounds on molecularly imprinted polymers (MIPs) using 1,1′-bi-2-naphthol (BINOL) as template. MIP prepared using 4-vinylpyridine as the functional monomer showed better chiral recognition for the template than the MIPs prepared using acrylamide, 2-(diethylamino)ethylmethacrylate and 2-vinylpyridine, respectively. 1H-NMR was used for comparison of the interactions between template and functional monomers. For chromatographic analysis the effects of mobile phase and temperature on the chiral separation were investigated. When 4-vinylpyridine was employed as the functional monomer, chiral separation of 1,1′-bi-2-naphthol and its analogues were studied. The MIP also demonstrated an ability to discriminate between enantiomers of structurally related compounds that had not been imprinted. The thermodynamic parameters of interactions between substrates and MIP in acetonitrile based mobile phase were investigated by the Van’t Hoff equation. In this study, the specific hydrogen-bonding interactions seemed to be the key factor to achieve chiral separation.






Similar content being viewed by others
References
Wulff G (1995) Angew Chem Int Ed Engl 34:1812–1832
Katz A, Davis ME (1999) Macromolecules 32:4113–41219
Ramström O, Ansell R (1998) Chirality 10:195–209
Piletsky SA, Alcock S, Turner APF (2001) Trends Biotechnol 19:9–12
Yang G, Yin J, Li Z, Liu H, Cai L, Wang D, Chen Y (2004) Chromatographia 59:705–708
Kempe M, Mosbach K (1995) J Chromatogr 694:3–13
Tto K, Tomita Y, Katsuki T (2005) Tetrahedron Lett 46:6083–6086
Krause K, Chankvetadze B, Okamoto Y, Blaschke G (1999) Electrophoresis 20:2772–2778
Sibrian-Vazquez M, Spivak DA (2004) J Am Chem Soc 126:7827–7833
Liu Z, Xu Y, Wang H, Yan C, Gao R (2004) Anal Sci 20:673–678
Brunkan NM, Gagne MR (2000) J Am Chem Soc 122:6217–6225
Ou J, Tang S, Zou H (2005) J Sep Sci 28:2282–2287
Hart BR, Rush DJ, Shea K (2000) J Am Chem Soc 22:460–465
Sabourin L, Ansell RJ, Mosbanch K, Nicholls IA (1998) Anal Commun 35:285–287
Sellergren B, Shea KJ (1995) J Chromatogr A 690:29–39
Yu C, Mosbanch K (1998) J Mol Recognit 11:69–74
Idziak I, Benrebouh A, Deschamps F (2001) Anal Chim Acta 435:137–140
Spivak DA, Simon R, Campbell J (2004) Anal Chim Acta 504:23–30
Allender CJ, Heard CM, Brain KR (1997) Chirality 9:238–242
Piletsky SA, piletska EV, Karim K, Freebairn KW, Legge CH, Turner APF (2002) Macromolecules 35:7499–7504
Dasgupta PK, Kephart TS (2000) Talanta 56:977–987
Li J, Carr PW (1996) Anal Chem 68:2857–2868
Lin JM, Nakagama T, Uchiyama K, Hobo T (1997) Biomed Chromatogr 11:298–302
Acknowledgments
This work was financed by the National Nature Science Foundation of China (No. 20202015) and the Research Foundation for the Doctoral Program of High Education of China (No. 20020558026).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Y., Liu, L., Li, L. et al. Chromatographic Separation of the Enantiomers of a Series of C2-Asymmetric Bi-Naphthyl Compounds by Molecularly Imprinted Polymers. Chroma 64, 393–397 (2006). https://doi.org/10.1365/s10337-006-0017-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0017-5