Abstract
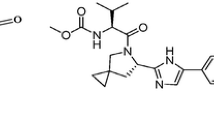
A rapid, simple and sensitive high-performance liquid chromatographic method (HPLC) has been developed to assay ritonavir in semisolid capsules. The HPLC analysis used a reversed-phase C8 (125 × 4.0 mm i.d., 5 μm particle size) analytical column and a mobile phase consisting of methanol and water (67:33, v/v), with UV detection at 210 nm. Specificity was evaluated using a photodiode array detector (PDA). The validation data showed that the assay is sensitive, specific and reproducible for determination of ritonavir in this dosage form. Calibration curves were linear from 100–300 μm L−1 (R2 ≥ 0.999). The accuracy of the method ranged from 98.8 to 102.0%. Mean inter- and intra-assay relative standard deviations (RSD) were less than 1.0%. The proposed method provided an accurate and precise analysis of ritonavir in soft capsules, requiring neither the use of a buffered mobile phase, nor the addition of amine modifiers.
Similar content being viewed by others
References
Sethi ML (2002) Antiviral Agents and Protease Inhibitors. In: Foye's Principles of Medicinal Chemistry, Williams DA, Lemke TL, Philadelphia
Tavares W (1996) Manual de Antibióticos e Quimioterápicos Antiinfecciosos, São Paulo
Raffanti S, Haas DW (2001) Antimicrobial Agents-Antiretroviral Agents. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics, Hardman JG, Limbird LE (eds) Gilman AG, USA
Abbott Laboratories, Norvir® (ritonavir capsules) Soft Gelatin / (ritonavir oral solution), North Chicago, IL 60064, U.S.A., Ref.: 03-5409-R20, Revised: January, 2005: http://www.norvir.com/pdf/norpi2a.PDF
Chen X, Kempf DJ, Li L, Sham HL, Vasavanonda S, Wideburg NE, Saldivar A, Marsh KC, McDonald E, Norbeck DW (2003) Bioorg Med Chem Lett 13:3657–3660
Korolkovas A (2004) Dicionário Terapêutico Guanabara, Rio de Janeiro
Murphy RL (2003) J Acquir Immune Defic Syndr 33:S33-S56.
FDA, HIV/AIDS Timeline Chronology of Significant Events, September, 2004: http://www.fda.gov/oashi/aids/miles.html
O'Neil MJ, Smith A, Heckelman PE, Budavari S (eds) (2001) The Merck Index: An Encyclopedia of Chemicals, Drugs & Biologicals, Whitehouse Station, NJ
Marsh KC, Eiden E, McDonald E (1997) J Chromatogr B Biomed Sci Appl 704:307–313
Hoetelmans RM, van Essenberg M, Profijt M, Meenhorst PL, Mulder JW, Beijnen JH (1998) J Chromatogr B Biomed Sci Appl 705:119–126
Frappier S, Breilh D, Diart E, Ba B, Ducint D, Pellegrin JL, Saux MC (1998) J Chromatogr B Biomed Sci Appl 714:384–389. DOI: 10.1016/s0378–4347(98)00220-5
Marzolini C, Telenti A, Buclin T, Biollaz J, Decosterd LA (2000) J Chromatogr B Biomed Sci Appl 740:43–58. DOI: 10.1016/s0378-4347(99)00573-3
Dailly E, Thomas L, Kergueris MF, Jolliet P, Bourin M (2001) J Chromatogr B Biomed Sci Appl 758:129–135. DOI: 10.1016/s0378-4347(01)00117-7
Sarasa-Nacenta M, López-Púa Y, Mallolas J, Blanco JL, Gatell JM, Carné X (2001) J Chromatogr B Biomed Sci Appl 757:325–332. DOI: 10.1016/s0378-4347(01)00172-4
Yamada H, Kotaki H, Nakamura T, Iwamoto A (2001) J Chromatogr B Biomed Sci Appl 755:85–89. DOI: 10.1016/s0378-4347(00)00617-4
Leibenguth P, Le Guellec C, Besnier JM, Bastides F, Macé M, Gaudet ML, Autret-Leca E, Paintaud G (2001) Ther Drug Monit 23:679–688
Simon VA, Thiam MD, Lipford LC (2001) J Chromatogr A 913:447–453. DOI: 10.1016/s0021-9673(00)01092-x
Villani P, Feroggio M, Gianelli L, Bártoli A, Montagna M, Maserati R, Regazzi MB (2001) Ther Drug Monit 23:380–388
Volosov A, Napoli KL, Soldin SJ (2001) Clin Biochem 34:285–290
Janoly A, Beyzac N, Favetta P, Gagneu MC, Bourhis Y, Coudray S, Oger I, Aulagner G (2002) J Chromatogr B Biomed Sci Appl 780:155–160
Ray J, Pang E, Carey D (2002) J Chromatogr B Biomed Sci Appl 775:225–230
Marzolini C, Béguin A, Telenti A, Schreyer A, Buclin T, Biollaz J, Decosterd LA (2002) J Chromatogr B Biomed Sci Appl 774:127–140. DOI: 10.1016/s1570-0232(02)00169-1
Titier K, Lagrange F, Péhourcq F, Edno-Mcheik L, Moore N, Molimard M (2002) Therapie 57:169–174
Tribut O, Arvieux C, Michelet C, Chapplain JM, Allain H, Bentue-Ferrer D (2002) Ther Drug Monit 24:554–562. DOI: 10.1097/01.FTD.0000017718.61918.93
Poirier JM, Robidou P, Jaillon P (2002) Ther Drug Monit 24:302–309
Chi JD, Jayewardene AL, Stone JA, Motoya T, Aweeka FT (2002) J Pharm Biomed Anal 30:675–684
Turner ML, Reed-Walker K, King JR, Acosta EP (2003) J Chromatogr B Biomed Sci Appl 784:331–341
Justesen US, Pedersen C, Klitgaard NA (2003) J Chromatogr B Biomed Sci Appl 783:491–500
Faux J, Venisse N, Le Moal G, Dupuis A, Bouquet S (2003) Chromatographia 58:421–426. DOI: 10.1365/s10337-003-0076-90009-5893/03/10
Droste JAH, Verweij-van Wissen CPWGM, D.M. Burger DM (2003) Ther Drug Monit 25:393–399
Walson PD, Cox S, Utkin I, Gerber N, Crim L, Brady M, Koranyi K (2003) Ther Drug Monit 25: 588–592
Frerichs VA, DiFrancesco R, Morse GD (2003) J Chromatogr B Biomed Sci Appl 787:393–403
Rentsch KM (2003) J Chromatogr B Biomed Sci Appl 788:339–350
The United States Pharmacopeia, 28th (2005), Rockville, MD
ICH (1994) Harmonised Tripartite Guideline, Text on Validation of Analytical Procedures, Q2A. In: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
ICH (1996) Harmonised Tripartite Guideline, Validation of Analytical Procedures: Methodology, Q2B. In: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
FDA (1994) Center for Drug Evaluation and Research. Reviewer Guidance: Validation of Chromatographic Methods, Rockville, MD
Bakshi M, Singh S (2002) J Pharm Biomed Anal 28:1011–1040
Rowe RC, Sheskey PJ, Weller PJ (eds) (2000) Handbook of Pharmaceutical Excipients, Washington
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dias, C., Rossi, R., Donato, E. et al. LC Determination of Ritonavir, a HIV Protease Inhibitor, in Soft Gelatin Capsules. Chroma 62, 589–593 (2005). https://doi.org/10.1365/s10337-005-0670-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-005-0670-0