Abstract
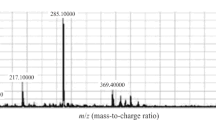
A simple, specific and sensitive high performance liquid chromatography-mass spectrometry (LC-MS) method for the determination of risperidone and its active metabolite 9-hydroxyrisperidone in human plasma has been developed and validated. The analytes were prepared through a single-step liquid-liquid extraction (LLE) procedure with the solvent methyl tert-butyl ether and quantitated by MS detection in the positive mode using selected ion monitoring (SIM). Each analytical run was completed within 9 min. Results showed that the LC-MS method enabled to detection of both compounds down to 0.1 ng.mL−1 (S/N > 3) and the linear range was 0.2–24 ng.mL−1, with the correlation coefficients above 0.99. At the concentration of 0.2, 0.5, 10 and 20 ng.mL−1, the inter-day and intra-day RSD were both below 15%. The method has been successfully used to support the routine therapeutic drug monitoring (TDM) and the pharmacokinetics study of risperidone.
Similar content being viewed by others
References
Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, Labelle A, Beauclair L, Amott W (1993) J Clin Psychopharmacol 13:25
Leysen JE, Gommeren W, Eens A, De Chaffoy de Courcelles D, Stoof JC, Jassen PA (1988) J Pharmacol Exp Ther 247:661
Heykants J, Huang ML, Mannens G, Meuldermans W, Snoeck E, Vanbeijsterveldt L, VanpeerA, Woestenborghs R (1994) J Clin Psychiatry 55:513
Mannens G, Human ML, Weuldermans W, Hendrickx J, Woestenborghs R, Heykants J (1993) Drug Metab Dispos 21:1134
Huang ML, Van Peer A, Woestenborghs R, De Coster R., Heykants J, Zylicz A, Visscher H, Jonkman J (1993) Clin Pharmacol Ther 54:252
Van Beijsterveldt L, Geerts R, Leysen J, Megens A, Van den Eynde H, Meuldermans W, Heykants J (1994) Psychopharmacology 114:53
Megents A, Awoutters F (1994) Drug Dev Res 33:399
Woestenborghs R, Geuens I, Lenoir H, Janssen C, Heykants J, in: Reid E, Wilson ID (Eds), Methodological Surveys in Biochemistry and Analysis, (1990) Royal Society of Chemistry 20:241
Nagasaki T, Ohkuo T, Sugawara K, Yasui N, Furukori H, Kaneko S (1999) J Chromatogr B 19:595
Le Moing JP, Edouard S, Levron JC (1993) J Chromatogr 614:333
Balant-Gorgia AE, Gex-Fabry M,Genent C, Balant LP (1998) Ther Drug Monit 21:105
Remmerie BMM, Sips LLA, De Vries R., De Jong J, Schothuis AM, Hooijschuur EWJ (2003) J Chromatogr B 783:461
Titier K, Bouchet S, Pehourcq F (2003) J Chromatogr B 788:179
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Jiao, Z., Yao, Z. et al. The Validation of an LC-MS Method for the Determination of Risperidone and its Active Metabolite 9-Hydroxyrisperidone in Human Plasma. Chroma 61, 245–251 (2005). https://doi.org/10.1365/s10337-005-0506-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-005-0506-y