Abstract
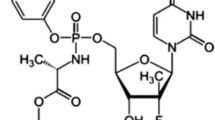
A sensitive, selective, precise and stability-indicating high-performance thin-layer chromatographic method of analysis of lamivudine both as a bulk drug and in formulations was developed and validated. The solvent system consisted of carbon tetrachloride – methanol – chloroform - acetonitrile (7.0: 3.0: 2.0: 1.5, v/v/v/v). Densitometric analysis of lamivudine was carried out in the absorbance mode at 275 nm. This system was found to give compact spots for lamivudine (R F value of 0.36 ± 0.02) following double development of chromatoplates with the same mobile phase. Lamivudine was subjected to acid and alkali hydrolysis, oxidation, dry heat and wet heat treatment and photo degradation. The drug undergoes degradation under acidic, basic conditions, oxidation, wet heat and photo degradation. Also the degraded products were well resolved from the pure drug with significantly different R F values. Linearity was found to be in the range of 50 – 1000 ng spot−1 with significantly high value of correlation coefficient. The linear regression analysis data for the calibration plots showed good linear relationship with r2 = 0.9994 ± 0.05 in the working concentration range of 300 ng spot−1 to 1000 ng spot−1. The mean value of slope and intercept were 0.11 ± 0.08 and 10.47 ± 1.21, respectively. The method was validated for precision, robustness and recovery. The limit of detection and quantitation were 15 ng spot−1 and 40 ng spot−1 respectively. As the method could effectively separate the drug from its degradation products, it can be employed as a stability indicating one. Moreover, the proposed HPTLC method was utilized to investigate the kinetics of acid degradation process. Arrhenius plot was constructed and activation energy was calculated.
Similar content being viewed by others
References
Van Leeuwen R, Katlama C, Kitchen V, Boucher CA, Tubiana R, McBride M, Ingrand D, Weber J, Hill A, McDade H (1955) J Infect Dis 171:1166
Pluda JM, Cooley TP, Montaner JS, Shay LE, Reinhalter NE, Warthan SN, Ruedy J, Hirst HM, Vicary CA, Quinn JB (1995) J Infect Dis 171:1438
Ingrand D, Weber J, Boucher CA, Loveday C, Robert C, Hill A, Cammack N (1995) AIDS 9:1323
Lewis LL, Venzon D, Church J, Farley M, Wheeler S, Keller A, Rubin M, Yuen G, Mueller B, Sloas M, Wood L, Balis F, Shearer GM, Brouwers P, Goldsmith J, Pizzo PA (1996) J Infect Dis 174:6
Coates JAV, Cammack N, Jenkinson HJ, Jowerr AJ, Jowett MI, Pearson BA, Penn CR, Rouse PL, Viner KC, Cameron JM (1992) Antimicrob. Agents Chemother 36:733
Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M (1995) New Engl. J Med 333:1657
Benhamou Y, Dohin E, Lunel-Fabiani F, Poynard T, Huraux JM, Katlama C, Opolon P, Gentilini M (1995) Lancet 345:396
Lai CL, Chien RN, Leung NWY, Chang TT, Guan R, Tai DI, Dent JC, Barber J, Stephenson SL, Gray DF (1998) New Engl. J Med 339:61
Larder BA, Kemp SD, Harrigan PR (1995) Science 269:696
Larder BA, Darby G, Richman DD (1989) Science 243:1731
Richman DD, Grimes JM, Lagakos SW (1990) J Acquir Immune Defic Syndr 3:743
Van Leeuwen R, Lange JM, Hussey EK, Donn KH, Hall ST, Harker AJ, Jonker PL, Danner SA (1992) AIDS 6:471
Guidelines for the Use of Antiretroviral Agents in HIV Infected Adults and Adolescents, US Department of Health and Human Services, January 2000
Morris DM, Selinger K (1994) J Pharm Biomed Anal 12:255
Harker AJ, Evans GL, Hawley AE, Morris DM (1994) J Chromatogr B 657:227
16Hsyu PH, Lloyd TL (1994) J Chromatogr B 655:253
Zhou XJ, Sommadossi JP (1997) J Chromatogr B 691:417
Hoetelmans RM, Profijt M, Meenhorst PL, Mulder JW, Beijnen JH (1998) J Chromatogr B 713:387–394
Kenney KB, Wring SA, Carr RM, Wells GN, Dunn JA (2000) J Pharm Biomed Anal 22:967
Pereira AS, Kenney KB, Cohen MS, Hall JE, Eron JJ, Tidwell RR, Dunn JA (2000) J Chromatogr B 742:173
Zheng JJ, Wu ST, Emm TA (2001) J Chromatogr B 761:195–201
Fan B, Steward JT (2002) J Pharm Biomed Anal 28:903–908
Simon VA, Thiam MD, Lipford LC (2001) J Chromatogr A 913:447–453
Uslu B, Ozakan SA (2002) Anal Chim Acta 466:175–185
Wring SA, O’Neill RM, Williams JL, Jenner WN, Daniel MJ, Gray MRD, Newman JJ, Wells GN, Sutherland DR (1994)J Pharm Biomed Anal 12:1573
ICH, Q1A Stability Testing of New Drug Substances and Products, International Conference on Harmonization, Genava, October 1993
Vuorela P, Rahko EL, Hiltunen R, Vuorela H (1994) J Chromatogr A 670:191–198
Ferenczi-Fodor K, Vigh Z, Nagy-Turák A, Renger B, Zeller M (2001) J AOAC Int 84:1265–1276
Ferenczi-Fodor K, Vigh Z (2001) Planar Chromatography-A Retrospective view from the third millennium (Sz. Nyiredy, edt), Springer Scientific Publisher, Budapest, 336–352
European Pharmacopoeia, Council of Europe, Strasbourg, 3rd ed., (1998) Suppl., (1999) pp. 5–7
Thin layer chromatography, Monograph 2.2.27, European Pharmacopoeia, Published by Council of Europe, 4.5 (2002) 3638
Mantovani G, Vaccari G, Dosi E, Lodi G (1998) Carbohydr Polym 37:263
Doner LW (2001) Chromatographia, 53:579
Sherma J, Fried B (1996) (Eds.), Handbook of Thin-Layer Chromatography, Marcel Dekker, New York, 2nd ed., pp. 129–148 and pp. 273–306
Renger B (1993) J AOAC Int 76:7–14
Abdelrahman AN, Karim EIA, Ibrahim KEE (1994) J Pharm Biomed Anal 12:205–208
Kaniou I, Zachariadis G, Kalligas G, Tsoukali H, Stratis J (1994) J. Liq. Chromatogr 17:1385–1398
Ponder GW, Stewart JT (1994) J Chromatogr A 659:177–183
Weins C, Hauck HE (1996) LC GC, 14:456–464
McCarthy KE, Wang Q, Tsai EW, Gilbert RE, Brooks MA (1998) J Pharm Biomed Anal 17:671–677
Jovanovic SE, Agbaba D, Zivanov-Stakic D, Vladimirov S (1998) J Pharm Biomed Anal 18:893–898
ICH, Q2A Validation of Analytical Procedure: Methodology, International Conference on Harmonization, Geneva, October, 1994
ICH, Q2B Validation of Analytical Procedure: Methodology, International Conference on Harmonization, Geneva, March, 1996
ICH Guidance on Analytical Method Validation, International Convention on Quality for the Pharmaceutical Industry, Toronto, Canada, September 2002
Sethi PD (1996) High Performance Thin Layer Chromatography, Quantitative Analysis of Pharmaceutical Formulations, CBS Publishers and Distributors, New Delhi
Garrett ER, Carper RF (1955), J Am Pharm Assoc Sci 44:515–521
Carstensen JT, Rhodes CT (2000) Drug Stability: Principles and Practices, 3rd edn, Revised and Expanded, Marcel Dekker Inc., Eastern Hemisphere Distribution, New York
Schyve PM, Prevost JA (1990) Psychiatr Clin North Am 13(1):61–67
Xiang Y, Wang B (1993) Chin J Univ Chem 8:34–38
Mahadik KR, Agrawal H, Kaul N, Paradkar AR (2003) J Pharm Biomed Anal 33:(4)545–552
Mahadik KR, Agrawal H, Kaul N, Paradkar AR (2003) Talanta 61:581–589
Mahadik KR, Agrawal H, Kaul N, Paradkar AR (2003) Drug Dev Ind Pharm 29(10):1119–1126
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaul, N., Agrawal, H., Paradkar, A. et al. The International Conference on Harmonisation Guidance in Practice: Stress Degradation Studies on Lamivudine and Development of a Validated Specific Stability-Indicating HPTLC Assay Method. Chromatographia 60, 213–221 (2004). https://doi.org/10.1365/s10337-004-0367-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-004-0367-9