Abstract
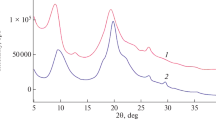
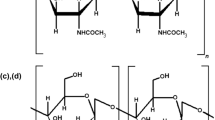
At the Institute of Polymer Chemistry and Physics we have investigated the isolation of chitin from a variety of raw materials—of Aral Sea crustaceans, the basidial fungus Pleurotus ostreatus, and waste from production of natural silk from the chrysalis of the silkworm Bombyx mori. The silk worm chrysalis, waste material from silk production, is a very valuable and readily available source of chitin, although new technology must be developed to enable use of the waste. Other raw materials suitable for isolation of chitin are also being investigated. Other subjects of interest are modification of chitin, preparation of chitosan from it, and interaction of these materials with transition metals ions, because water-soluble polymer–metal complexes of known metal-ion content have medical and agricultural applications. Formation of complexes between chitosan and transition metal ions (Co, Ni, Cu, Mn) has been investigated. Differences between the spectra of chitosan and its metal complexes are all in the region of the stretching and deformation vibrations of the amide bonds (amide-I, amide-II, and amide-III). This is because the synthesis of chitosan–metal complexes was conducted in acid media, in which the chitosan amino group is protonated, so the preferred interaction takes place with the amide bond of chitosan. Noticeable changes of amide-I and amide-II intensities are observed, depending on the metal ion content; this changes can be used for estimation of the metal content in the samples. It has been shown that in the samples synthesized the metal ions are distributed fairly uniformly along the polymer chains. The composition and structures of the complexes formed, the conditions needed to include the different functional groups of the polymer in the coordination process, and the dynamics of macromolecular tangling behavior in the process of metal-ion binding were determined. This enables prediction of the behavior of chitosan–metal-ion systems under conditions of use.
Similar content being viewed by others
References
Feofilova EP, Tereshina VM (1999) Prospective natural sources of chitin. Fifth All-Russian Conf. on New Perspectives in Chitin and Chitosan Investigation, Schelkovo, Moscow
Nemtsov SV, Zueva SYu, Khismatullin RG (2001) The bee as a potential source of chitosan. Sixth All-Russian Conf. on New Perspectives in Chitin and Chitosan Investigation, Schelkovo, Moscow
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at: International Symposium on Separation and Characterization of Natural and Synthetic Macromolecules, Amsterdam, The Netherlands, February 5–7, 2003
Rights and permissions
About this article
Cite this article
Rashidova, S., Milusheva, R., Voropaeva, N. et al. Isolation of Chitin from a Variety of Raw Materials, Modification of the Material, and Interaction its Derivatives with Metal Ions. Chromatographia 59, 783–786 (2004). https://doi.org/10.1365/s10337-004-0290-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-004-0290-0