Abstract
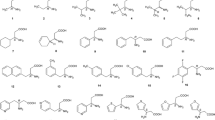
A variety of amino acids (primary and secondary), peptides and amino alcohols are pre-column phenyl isocyanated in alkaline medium and enantioresolved on the naphthylethylcarbamated β-cyclodextrin (i.e., RN- and SN-β-CD) bonded chiral phases (CSPs) using the acetonitrile-based mobile phase and on a native β-cyclodextrin (β-CD) phase for comparison. The resolution is believed to be a result of the hydrogen bonding between the secondary hydroxyl groups of cyclodextrin and the functional groups of analyte and is enhanced as the amino and the carboxyl groups are attached to the stereogenic center of analyte. Also, the enhancement is observed if the steric hindrance between the side-chain group of amino acid and the chiral selector exists. However, the resolution is deteriorated in the case that the side-chain group close to the stereogenic center of amino acid becomes bulky or is capable of forming hydrogen bonding with chiral selector. The aromatic moiety of the tagging reagent not only contributes the retention, but also benefits the resolution in some cases on the RN- and SN-β-CD phases through π-π interaction. The resolution is either not observed or unsatisfactory in the reversed-phase mode.
Similar content being viewed by others
References
Shinbo T, Yamaguchi T, Nishimura K, Suguira M (1987) J Chromatogr 405:145–513
Hilton M, Armstrong DW (1991) J Liq Chromatogr 14:9–28
Hilton M, Armstrong DW (1991) J Liq Chromatogr 14:3673–3683
Gibitz G, Juffman E, Jelleng W (1982) Chromatographia 16:103–106
Davankov VA, Semechkin AV (1977) J Chromatogr 141:313–353
Armstrong DW, Yang X, Han SM, Menges RA (1987) Anal Chem 59:2594–2596
Berthod A, Liu Y, Bagwill C, Armstrong DW (1996) J Chromatogr A 731:123–137
Sanger F (1945) Biochem J 45:563–567
Zukowski J, Pawlowska M, Armstrong DW (1992) J Chromatogr 623:33–41
Zukowski J, Pawlowska M, Nagatkina M, Armstrong DW (1992) J Chromatogr 629:169–179
Chang SC, Wang LR, Armstrong DW (1992) J Liq Chromatogr 15:1411–1429
Pawlowska M, Chen S, Armstrong DW (1993) J Chromatogr 641:257–265
Chen S (1996) J Chin Chem Soc 43:45–51
Chen S (1996) J Chin Chem Soc 43:503–506
Chen S (1999) J Chin Chem Soc 4:239–244
Chen S (1997) Chin Pharm J 43:51–60
Chen S (2004) Amino Acids (accepted)
Chen S (2003) J Liq Chromatogr 26:3475–3495
Armstrong DW, Chen, S, Chang, C, Chang, S (1992) J Liq Chromatogr 15:545–556
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S. The HPLC Enantioresolution of Phenyl Isocyanated Amino Acids, Peptides and Amino Alcohols on Cyclodextrin Bonded Phases Using the Acetonitrile-Based Mobile Phase. Chromatographia 59, 697–703 (2004). https://doi.org/10.1365/s10337-004-0282-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-004-0282-0