Abstract
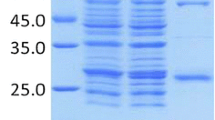
Human oestrogen receptor (alpha) ligand binding domain (hER-LBD) was expressed in E. coli and isolated using a novel approach. The solubilised recombinant receptor had the expected biological activity in terms of ligand binding affinity and selectivity, indicating the potential for use in the proposed receptor affinity chromatography (RAC) application. Subsequent covalent binding of hER-LBD to agarose support provided an affinity matrix capable of selective binding of oestrogenic ligands, with a capacity for 17β-oestradiol of ∼6 ng/mL wet gel. In initial studies, a yield of ∼75% of bound ligand from the affinity matrix was obtained by elution with aqueous ethanol. Immobilised hER-LBD eluted with ethanol retained the majority of its capability to bind 17β-oestradiol (E2), indicating the possibility of reuse of the receptor matrix. In ligand-receptor displacement studies, using [3H]E2-saturated immobilised hER-LBD, direct extraction of the xenoestrogen 2′,3′,4′,5′-tetrachloro-4-biphenylol (TeCBol) from a model food (aqueous gelatin solution) was inhibited at the highest concentration of gelatin tested (1%), however, prior precipitation and extraction with ethanol enabled dose dependent binding of TeCBol. The present studies thus provide preliminary proof of principle for the application of hER-LBD for the purpose of RAC and for the generic extraction of oestrogens and xenoestrogens from biological matrices.
Similar content being viewed by others
References
Geise R (2003) J Chromatogr A 1000:401–12
Lopez de Alda MJ, Barcelo D (2001) Fresenius J Anal Chem 371:437–447
Coffer A, Cavailles V, Knowles P, Pappin DJ (1996) Steroid Biochem Molec Biol 58:467–477
Seilestad DA, Carlson KE, Katzenellenbogen JA, Krushner PJ, Greene GL (1995) Molec Endocrinol 9:647–658
Kim SB, Ozawa T, Umezawa Y (2003) Anal Sci 19:499–504
Bauer ER, Bitsch N, Brunn H, Sauerwein H, Meyer HH (2002) Chemosphere 46:1107–1115
Allan GF, Hutchins A, Clancy J (1999) Anal Biochem 275:243–247
Murata M, Nakayama M, Irie H, Yakabe K, Fukuma K, Katayama Y, Maeda M (2001) Anal Sci 17:387–390
Usami M, Mitsunaga K, Ohno Y (2002) J Steroid Biochem Mol Biol 81:47–55
Hock B, Seifert M, Kramer K (2002) Biosens Bioelectron 17:239–249
Ikeda M, Watanabe S, Kusaka T (1988) Steroids 52:187–203
Ikeda M, Kusaka T (1989) Steroids 54:217–226
Hutchens TW, Li CM (1990) J Mol Recognit 3:174–179
Author information
Authors and Affiliations
Corresponding author
Additional information
British Crown Copyright and Crown user rights are reserved for this work of M.F. Byford and M.J. Sauer
In Honour of Professor Edward Houghton: A Celebration of 30 Years in Racing Chemistry
Rights and permissions
About this article
Cite this article
Byford, M., Sauer, M. Application of Oestrogen Receptor Ligand Binding Domain to the Generic Isolation of Oestrogens by Receptor Affinity Chromatography. Chromatographia 59 (Suppl 1), S123–S130 (2004). https://doi.org/10.1365/s10337-003-0246-4
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1365/s10337-003-0246-4