Abstract
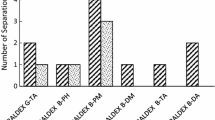
Analytical HPLC methods for derivatized amylose chiral stationary phases were developed for the direct enantioseparation of substituted [1-(imidazo-1-yl)-1-phenylmethyl)] benzothiazolinone and benzoxazolinone derivatives with one stereogenic center. These analogues of fadrozole constitute new potent nonsteroidal inhibitors of aromatase (P450 arom.). The separations were made using normal phase methodology with mobile phase consisting of n-hexane-alcohol (ethanol, 1-propanol or 2-propanol) in various proportions, and a silica-based amylose tris-3,5-dimethylphenylcarbamate (Chiralpak AD), or tris-(S)-1-phenylethylcarbamate (Chiralpak AS). The effects of concentration of various aliphatic alcohols in the mobile phase were studied. Baseline separation (R s > 1.5) was easily obtained in all cases, ethanol being often the more interesting modifier. The effects of structural features of the solutes along with the temperature of the column on the discrimination between the enantiomers were examined for different mobile phase compositions.
Similar content being viewed by others
References
Segaloff A (1978) Hormones and mammary carcinogens. In: Advances in Research and Treatment, Experimental Biology Plenum (eds) WL McGuire, New York
Persiani S, Broutin F, Cicioni P, Stefanini P, Strolin Benedetti M (1996) Eur J Pharm Sci 4:331–340
Enguehard C, Renou J.L, Allouchi H, Leger JM, Gueiffier A (2000) Chem Pharm Bull 48:935–940
Auvray P, Moslemi S, Sourdaine P, Galopin S, Seralini GE, Enguehard C, Dallemagne P, Bureau R, Sonnet P, Rault S (1998) Eur J Med Chem 33:451–462
Njar VCO, Brodie AMH (1999) Drugs 58:233–255
Feutrie ML, Bonneterre J (1999) Bull Cancer 86:821–827
Sonnet P, Guillon J, Enguehard C, Dallemagne P, Bureau R, Rault S, Auvray P, Moslemi S, Sourdaine P, Galopin S, Seralini GE (1998) Bioorg Med Chem Lett 8:1041–1044
Marchand P, Le Borgne M, Palzer M, Le Baut G, Hartmann RW (2003) Bioorg Med Chem Lett 13:1553–1555
Le Borgne M, Marchand P, Delevoye-Seiller B, Robert J, Le Baut G, Hartmann RW, Palzer M (1999) Bioorg Med Chem Lett 9:333–336
Karjalainen A, Kalapudas A, Södervall M, Pelkonen O, Lammintausta R (2000) Eur J Pharm Sci 11:109–131
Francotte E, Junker-Buchheit A (1992) J Chromatrogr 576:1–45
Furuta R, Nakazawa H (1992) J Chromatogr 625:231–235
Morin N, Guillaume YC, Peyrin E, Rouland JC (1998) Anal Chem 70:2819–2826
Aboul-Enein HY, Ali I (2001) Chromatographia 54:200–202
Balmér K, Persson BA, Lagerström PO (1994) J Chromatrogr A 660:269–273
Shibukawa A, Nakagawa T (1989) In: Chiral separations by HPLC: Krstulovic AM (eds) Ellis Horwood
Okamoto Y, Kaida Y (1994) J Chromatogr A 666:403–419
Liu G, Goodall D.M, Hunter AT, Massey PR (1994) Chirality 6:290–294
Daicel Chemical Industries, Instruction Manual for Chiralpak AS, Baker France Daicel Chemical Industries, Instruction Manual for Chiralpak AD, Baker France
Kunath A, Theil F, Wagner J (1994) J Chromatogr A 684:162–167
Okamoto Y, Yashima E (1998) Angew Chem In Ed 37:1020–1043
Dingenen J (1994) In: A Practical Approach to Chiral Separations by Liquid Chromatography (eds) Subramanian G, New-York
Francotte E (2001) J Chromatogr A 906:379–397
Ferretti R, Gallinella B, La Torre F, Zanitti L (1998) Chromatographia 47:649–654
Spitzer T, Yashima E, Okamoto Y (1999) Chirality 11:195–200
Aboul-Enein HY, Ali I, Schmid MG, Jetcheva V, Gecse O, Gübitz G (2001) Anal Lett 34:1107–1115
Messina F, Botta M, Corelli F, Paladino A (2000) Tetrahedron Asymmetry 11:4895–4901
Aboul-Enein HY, Ali I, Gübitz G, Simons C, Nicholls PJ (2000) Chirality 12:727–733
Ferretti R, Gallinella B, La Torre F, Turchetto L (1997) J Chromatogr A 769:231–238
Quaglia MG, Bossù E, Porreta GC, Biava M, Fioravanti R, Romanelli L, Leonardi A (1996) J Chromatogr A 729:1–4
Alfredson TV, Towne R, Elliot M (1996) J Liq Chrom Rel Techno 19:1653–1668
Furet C, Batzl C, Bhatnagar A, Francotte E, Rihs G, Lang M (1993) J Med Chem 36:1393–1400
Pepper C, Smith HJ, Banell KJ, Nicholls PJ, Hewlins MJE (1994) Chirality 6:400–406
Cirilli R, Costi R, Di Santo R, Ferretti R, La Torre F, Angioletta L, Micocci M (2002) J Chromatogr A 942:107–114
O’Brien T, Crocker L, Thompson R, Thompson K, Toma PH, Conlon DA, Feibush B, Moeder C, Bicker G, Grinberg N (1997) Anal Chem 69:1999–2007
Wainer IW, Alembik MC, Smith E (1987) J Chromatogr 388:65–74
Wainer IW, Stiffin RM (1987) J Chromatogr 411:139
Kunath A, Theil F, Jähnisch K (1996) J Chromatogr A 728:249–257
Wainer IW, Alembik MC (1986) J Chromatogr 358:85–93
Balmér K, Lagerström PO, Persson BA (1992) J Chromatogr 592:331–337
Küsters E, Spöndlin C (1996) J Chromatogr A 737:333–337
Booth TD, Wainer IW (1996) J Chromatogr A 741:205–221
Kazusaki M, Kawabata H, Matsukura H (2000) J Liq Chrom Rel Technol 23:2937–2946
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danel, C., Foulon, C., Park, C. et al. Chiral Resolution of the Enantiomers of New Aromatase Inhibitors by Liquid Chromatography on Amylose Stationary Phases. Chromatographia 59, 181–188 (2004). https://doi.org/10.1365/s10337-003-0167-7
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1365/s10337-003-0167-7