Abstract
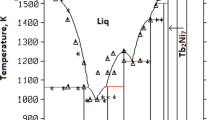
The Gibbs energy of formation of the solution and compound phases in the Nb-Sn system were derived from an optimization procedure using all the available experimental thermodynamic and phase diagram data. The thermodynamic description of the intermetallic compounds was made using a sublattice model, while a Redlich-Kister polynomial was used for the solution phases.
Similar content being viewed by others
Cited References
O. Redlich and A. Kister, “Algebraic Representation of Ther-modynamic Properties and the Classification of Solutions,”Ind. Eng. Chem., 40, 345–348 (1948).
C. Wagner,Thermodynamics of Alloys, Addison Wesley, Cambridge, MA, (1952).
B.T. Matthias, T.H. Geballe, S. Geller, and E. Corenzwit,Phys. Rev., 95, 1435(1954).
M.I.T. Massachusetts Institute of Technology 1961-1962,Ann. Rep. Res. Mater. Sci. Eng., 136, (April 1962).
S.B. Reed, H.C. Gatos, W.J. LaFleur, and J.T. Roody,Metallurgy of Advanced Electronic Materials, G.E. Brock, Ed., Interscience, New York, 71–87 (1962).
L.L. Wyman, J.R. Cuthill, G.A. Moore, J.J. Park, and H. Yakowitz,J. Res. Nat. Bur. Stand., 66A, 351–358 (1962).
G. Ellis and H.A. Wilhem,J.Less-Common Met., 7, 67–83(1964).
R.E.Enstrom, G.W. Pearsall, and J. Wulff,J. Met.,97 (Jan 1964).
L.J. Vieland,RCARev., 25, 366(1964).
J.R. Ogren, T.G. Ellis, and J.F. Smith,Acta Crystallogr., 18, 968 (1965).
J.H.N. van Vucht, DJ. Van Ooijen, and H. A.C. Bruning,Philips Res.Rep., 20, 136–161 (1965).
J.R Charlesworth, I. Macphail, and P.E. Madsen,J. Mater. Sci., 5, 580–603(1970).
A.A. Matsokova and B.G. Lazarev,Fiz. Met. Metalloved., 35(1), 148–156(1973).
G.A.Malets,Vestn. Akad. NavukB. SSR, Ser. Khim. Navukl, 126 (1975).
A.R. Miedema, P.F. Châtel, and ER. de Boer,Physica, 100B, 1 (1980).
R.A. Schiffman and D.M. Bailey,High Temp. Sci, 15, 165–177 (1982).
B. Sundman, B. Jansson, and J.O. Anderson,Calphad, 2(9), 153 (1985).
T.B.Massalski, J.L.Murray, L.H. Bennett, and H. Baker, Ed.,Binary Alloy Phase Diagrams, American Society For Metals, Metals Park, OH (1986).
F.de Boer, R. Boom, W.C. Mattens, A.R. Miedema, and A.K. Niessen, “Cohesion in Metals,”Transition Metal Alloys, North Holland, Amsterdam, 390–401 (1988).
A.T. Dinsdale,Calphad,15(4) (1991).
A. Servant, C. Gueneau, and I. Ansara,J. Alloy. Compd., 220, 19–26(1995).
I. Ansara, T.G. Chart, A. Fernandez Guillermet, F.H. Hayes, U.R. Kattner, D.G. Pettifor, N. Saunders, and K. Zeng, “Workshop on Thermodynamic Modeling of Solutions and Alloys,” Scholb Ringberg (10-16 March 1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toffolon, C., Servant, C. & Sundman, B. Thermodynamic assessment of the Nb-Sn system. JPE 19, 479 (1998). https://doi.org/10.1361/105497198770341978
Received:
Revised:
DOI: https://doi.org/10.1361/105497198770341978