Abstract
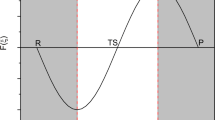
Proton transfer in carbonic anhydrase II has been studied at the B3LYP/6-31G(D) level. The active site model consists of the zinc ion, four histidine residues, two threonine residues, and three water molecules. Our calculations showed that the proton of the zinc-bound water molecule could be transferred to the nearest water molecule and an intermediate containing H3O+ is then formed. The intermediate is only 1.3 kJ·mol−1 above the reactant complex, whereas the barrier height for the proton transfer is about 8.1 kJ·mol−1.
Similar content being viewed by others
References
Copeland, R. A., Chan, S. I., Proton translocation in proteins, Annu. Rev. Phys. Chem., 1989, 40: 6711–698.
Silverman, D. N., Lindskog, S., Rate of exchange of water from the active site of human carbonic anhydrase C., Acc. Chem. Res., 1988, 21(1): 33–36.
Christianson, D. W., Fierke, C. A., Carbonic anhydrase: evolution of the zinc binding site by nature and by design, Acc. Chem. Res., 1996, 29(7): 331–339.
Silverman, D. N., Tu, C. K., Lindskog, S. et al., The catalytic mechanism of carbonic anhydrase: implications of a rate-limiting protolysis of water, J. Am. Chem. Soc., 1979, 101(22): 6734–6740.
Duda, D., Tu, C. K., Qian, M. Z. et al., Structural and kinetic analysis of the chemical rescue of the proton transfer function of carbonic anhydrade II, Biochem., 2001, 40(6): 1741–1748.
Hakansson, K., Carlsson, M., Svensson, L. A. et al., Structure of native and Apro carbonic anhydrase II and structure of some of its anion-ligand complexes, J. Mol. Biol., 1992, 227(4): 1192–1204.
Chen, H., Li, S. H., Jiang, Y. S., A density functional theory study on the intramolecular proton transfer in the enzyme carbonic anhydrase, J. Phys. Chem. A, 2003, 107(23): 4652–4660.
Cui, Q., Karplus, M., Is a “Proton Wire” concerted or stepwise? A model study of proton transfer in carbonic anhydrase, J. Phys. Chem. B, 2003, 107(4): 1071–1078.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhang, L., Xie, D. Density functional theory study of proton transfer in carbonic anhydrase. Chin. Sci. Bull. 50, 2557–2559 (2005). https://doi.org/10.1360/982005-485
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1360/982005-485