Abstract
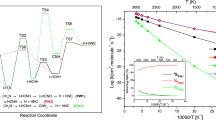
We report ab initio UMP2 calculations of the reaction of CN with HNCO using 6-311G(d,p) basis sets. The obtained results show that the reaction has two product channels: HNCO+CN→HCN+NCO (1) and HNCO+CN→HNCN+CO (2). Channel (1) is a hydrogen abstraction reaction, which is a concerted process. The calculated potential energy barrier is 20.80 kJ/mol at UMP2(full)/6-311G(d,p) level. In the range of reaction temperature (1000-2100 K), the conventional transition theory rate constant for channel (1) ranges from 0.32×10−11 to 6.9×10−11cm3· mol−1· s−1, which is close to the experimental value. Channel (2) is a stepwise reaction involving an intermediate during the process of reaction. The UMP2(full)/6-311G(d,p) potential energy barrier is 83.42 kJ/mol for the rate-controlling step, which is much higher than that of channel (1).
Similar content being viewed by others
References
Zyrianov, M., Droz-Georget, Th., Reisler, H. et al., Competitive photodissociation channels in jet-cooled HNCO: Thermochemistry and near-threshold predissociation, J. Chem. Phys., 1996, 105(18): 8111–8116.
Mertens, J. D., Kohse-Honghans, K., Bowman, C. T. et al., A shock tube study of H+HNCO→NH2+CO, Int. J. Kinet., 1991, 23: 655–667.
Xu Zhenfen, Sun Chia-Chung, Theoretical study on the reaction path and variational rate constant of the reaction HNCO+NH→ NH2+NCO, J. Phys. Chem. (A1), 1998, 102: 1194–1199.
Xu Zhenfen, Sun Chia-Chung, Ab initio study on reaction path and rate constant of the hydrogen atom abstraction reaction HNCO+N→NCO+NH, J. Mol. Struct. (Theochem.), 1999, 459: 37–46.
Ji, Y. Q., Feng, W. L., Ab initio study on the mechanism of reaction HNCO+NH2, Science in China, Ser. B, 2002, 45(4): 365–372.
Ji, Y. Q., Feng, W. L., MP2 and QCISD study of hydrogen transfer reaction path of the reaction HNCO with Carbon-Hydrogen radicals CHx (x=1-3), Acta Chimica Sinica, 2002, 60(7): 1167–1172.
Tsang, W., Chemical kinetic data base for propellant combustion, J. Phys. Chem. Ref. Data, 1992, 21(4): 753–791.
Head-Gordon, M., Pople, J. A., Frisch, M. J., MP2 energy evaluation by direct methods, Chem. Phys. Lett., 1988, 153: 503–509.
Frisch, M. J., Trucks, G. W., Pople, J. A. et al.,Gaussian 94, Pittsburgh, PA: Gaussian, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Zhen, Z. & Liu, X. Ab initio study of the reaction path of the reaction HNCO+CN. Sc. China Ser. B-Chem. 48, 279–285 (2005). https://doi.org/10.1360/04yb0051
Received:
Issue Date:
DOI: https://doi.org/10.1360/04yb0051