Abstract
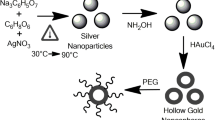
Gold nanoshells were prepared by an easy wet-chemical method, with Ag nanoparticles used as the templates. Transmission electron microscopy (TEM) indicated that the shells were sphere in shape, the size was homogenous, being about 20 nm, and no hard agglomerates were observed. The plasma resonance absorption of gold was tuned from visible to near-infrared (NIR) with the increased volume of HAu11CI4. The temperature grads (ΔT) of the gold nanoshell hydrosol under the exposure of an 808-nm optical fiber laser with different power densities were measured. The highest Δt was 30δC (5 W/cm2, irradiation area was 2 cm2). This kind of gold nanoshell hydrosol is a promising material to be used in biomedicine such as photothermal cancer therapy, and its special photothermal convert property will photothermally trigger drug release.
Similar content being viewed by others
References
Sun, Y., Xia, Y., Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium, J. Am. Chem. Soc, 2004, 126(12): 3892–3901.
Sun, Y, Gates, B., Mayers, B. et al., Crystalline silver nanowires by soft solution processing, Nano Lett., 2002, 2(2): 165–168.
Xia, Y, Yang, P., Chemistry and physics of nanowires, Adv. Mater., 2003, 15(5): 351–352.
Kim, F., Song, J. H., Yang, P., Photochemical synthesis of gold nanorods, J. Am. Chem. Soc, 2002, 124(48): 14316–14317.
Sun, Y, Mayers, B., Xia, Y, Transformation of silver nanospheres into nanobelts and triangular nan opiates through a thermal process, Nano Lett, 2003, 3(5): 675–679.
Maillard, M., Giorgio, S., Pileni, M., Silver nanodisks, Adv. Mater., 2002, 14(15): 1084–1086.
Jin, R., Cao, Y, Mirkin, C. A. et al., Photoinduced conversion of silver nanospheres to nanoprisms, Science, 2001, 294: 1901–1903.
Sun, Y, Xia, Y, Shape-control led synthesis of gold and silver nanoparticles, Science, 2002, 298: 2176–2179.
Sun, Y, Xia, Y, Increased sensitivity of surface plasmon resonance of gold nanoshells compared to that of gold solid colloids in response to environmental changes, Anal. Chem., 2002, 74(20): 5297–5305.
Hirsch, L. R., Stafford, R. J., Bankson, J. A. et al., Nanoshellmediated near-infrared thermal therapy of tumors under magnetic resonance guidance, Proc. Natl. Acad. Sci. U.S.A., 2003, 100(23): 13549–13554.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Song, H., Yu, L. et al. Wet-chemical synthesis and characteristics of Au nanoshell. Sc. China Ser. B-Chem. 48, 431–435 (2005). https://doi.org/10.1360/042004-101
Received:
Issue Date:
DOI: https://doi.org/10.1360/042004-101