Abstract
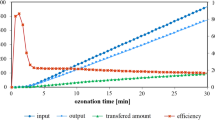
Aqueous solution of p-nitrophenol (PNP) was treated continuously by microwave assisted wet oxidation while flowing through a granular activated carbon (GAC) fixed bed. PNP was pre-adsorbed onto GAC prior to being put into the reactor so as to prevent PNP adsorption on GAC during microwave irradiation. PNP solutions with different initial concentration (218.6 mg/L and 1200 mg/L) were treated under conditions of microwave power 500 W, liquid flow 6.4 mL/min and air flow 40 mL/min or 60 mL/min. The results indicated that the removal of PNP was higher than 90% and more than 65% PNP was mineralized. Phenol, nitrobenzene, hydroquinone and benzoquinone occurred as course products during the operation process, which were degraded further. The biodegradability of the outflow was improved greatly by microwave assisted wet oxidation.
Similar content being viewed by others
References
Spain, J. C., van Veld, P. A., Monti, C. A. et al., Comparison of p-nitrophenol biodegradation in field and laboratory test systems, Applied and Environmental Microbiology, 1984, 48: 944–950.
Zaidi, B. R., Imam, S. H., Inoculation of microorganisms to enhance biodegradation of phenolic compounds in industrial wastewater: isolation and identification of three indigenous bacterial strains, Journal of General and Applied Microbiology, 1996, 42: 249–256.
Bhushan, B., Chauhan, A., Samanta, S. K. et al., Kinetics of biodegradation of p-nitrophenol by different bacteria, Biochemical and Biophysical Research Communications, 2000, 274: 626–630.
Bhatti, Z. I., Toda, H., Furukawa, K., p-Nitrophenol degradation by activated sludge attached on nonwovens, Water Research, 2002, 36: 1135–1142.
Rogers, K. R., Van Emon, J. M., Immunoassay for p-nitrophenol in urine, EPA Report, 1993, EPA/600/A-93/074, USA.
U.S. Environmental Protection Agency, Water Quality Criteria, 1976, U.S. Environmental Protection Agency, Washington, DC.
UK MOD, Defence standardization website, www.dstan.mod.uk/dsmain.htm.
Leung, K. T., Tresse, O., Aerrampalli, D. et al., Mineralization of p-nitrophenol by pentachlorophenol-degrading Sphingomonas spp., FEMS Microbiology Letters, 1997, 155: 107–114.
Hanne, L. F., Kirk, L. L., Appel, S. M. et al., Degradation and induction specificity in actinomycetes that degrade p-nitrophenol, Applied and Environmental Microbiology, 1993, 59: 3505–3508.
Jain, R. K., Dreisbach, J. H., Spain, J. C., Biodegradation of p-nitrophenol via 1,2,4-benzenetriol by an arthrobacter, Applied and Environmental Microbiology, 1994, 60: 3030–3032.
Spain, J. C., Gbison, D. T., Pathway for biodegradation of p-nitrophenol in a Moraxella sp., Applied and Environmental Microbiology, 1991, 57: 812–819.
Moreno-Castilia, C., Rivera-Utrilia, J., López-Ramón, M. V. et al., Adsorption of some substituted phenols on activated carbons from a bituminous coal, Carbon, 1995, 33: 845–851.
Chern, J. M., Chien, Y. W., Adsorption of nitrophenol onto activated carbon: isotherms and breakthrough curves, Water Research, 2002, 36: 647–655.
Chern, J. M., Chien, Y. W., Competitive adsorption of benzoic acid and p-nitrophenol onto activated carbon: isotherm and breakthrough curves, Water Research, 2003, 37: 2347–2356.
Gadekar, P. T., Mukkolath, A. V., Tiwari, K. K., Recovery of nitrophenols from aqueous solutions by a liquid emulsion membrane system, Separation Science and Technology, 1992, 27: 427–445.
Tompkins, C. J., Michaels, A. S., Peretti, S. W., Removal of p-nitrophenol from aqueous solution by membrane-supported solvent extraction, Journal of Membrane Science, 1992, 75: 277–292.
Dieckmann, M. S., Gray, K. A., A comparison of the degradation of 4-nitrophenol via direct and sensitized photocatalysis in TiO2 slurries, Water Research, 1996, 30: 1169–1183.
Bandara, J., Kiwi, J., Pulgarin, C. et al., Catalytic oxidation and photo-oxidation of nitrophenols by strong oxidants generated in situ via CuO-aerogel, Journal of Molecular Catalysis A: Chemical, 1996, 111: 333–339.
Kiwi, J., Pulgarin, C., Peringer, P., Effect of Fenton and photo-Fenton reactions on the degradation and biodegradability of 2 and 4-nitrophenols in water treatment, Applied Catalysis B: Environmental, 1994, 3: 335–350.
Hoffmann, M. R., Hua, I., Höchemer, R., Application of ultrasonic irradiation for the degradation of chemical contaminants in water, Ultrasonics Sonochemistry, 1996, S163–S172.
Oliviero, L., Barbier, J., Jr., Duprez, D., Wet air oxidation of nitrogen-containing organic compounds and ammonia in aqueous media, Applied Catalysis B: Environmental, 2003, 40: 163–184.
Deiber, G., Foussard, J. N., Debellefontaine, H., Removal of nitrogenous compounds by catalytic wet air oxidation Kinetic study, Environmental Pollution, 1997, 96: 311–319.
Edelstein, W. A., Iben, I. E. T., Mueler, O. M. et al., Radio frequency ground heating for soil remediation: science and engineering, Environmental Progress, 1994, 13: 247–252.
Adhikari, B., De D., Maiti, S., Reclamation and recycling of waste rubber, Progress in Polymer Science, 2000, 25: 909–948.
Chen, T. T., Dutrizac, J. E., Haque, K. E. et al., The relative transparency of minerals to microwave radiation, Canadian Metallurgical Quarterly, 1984, 123: 349–351.
Xia, L. X., Lu, S. W., Cao, G. Y., Demulsification of emulsions exploited by enhanced oil recovery system, Separation Science and Technology, 2003, 38: 4079–4094.
Cha, C. Y., Kim, D. S., Microwave induced reactions of sulfur dioxide and nitrogen oxides in char and anthracite bed, Carbon, 2001, 39: 1159–1166.
Menendez, J. A., Menendez, E. M., Garcia, A. et al., Thermal treatment of active carbons: a comparison between microwave and electrical heating, Journal of Microwave Power and Electromagnetic Energy, 1999, 34: 137–143.
Jou, C. H., Tai, H. S., Application of granulated activated carbon packed-bed reactor in microwave radiation field to treat BTX, Chemosphere, 1998, 37: 685–698.
Tai, H. S., Jou, C. H., Application of granular activated carbon packed-bed reactor in microwave radiation field to treat phenol, Chemosphere, 1999, 38: 2667–2680.
Abramovitch, R. A., Capracotta, M., Remediation of waters contaminated with pentachlorophenol, Chemosphere, 2003, 50: 955–957.
Liu, X. T., Quan, X., Bo, L. L. et al., Simultaneous pentachlorophenol decomposition and granular activated carbon regeneration assisted by microwave irradiation, Carbon, 2004, 42: 415–422.
Zou, Z. B., Fu, D. F., Zhang, L. et al., Removal of sulphe salicylicacid pollutants by microwave irradiation, Environmental Pollution and Control (in Chinese), 1999, 21: 22–24.
Park, M., Komarneni, S., Roy, R., Microwave-hydrothermal decomposition of chlorinated organic compounds, Materials Letters, 2000, 43: 259–263.
Wang, J. C., Xue, D. M., Quan, X. et al., Research on treating reactive brilliant blue KN-R solution by microwave radiation, Journal of Environmental Sciences (in Chinese), 2001, 21: 628–630.
Zhang, Y. B., Quan, X., Xue, D. M. et al., Stability for treatment of dye-containing wastewater using microwave assisted catalysis reactor, China Environmental Science (in Chinese), 2002, 22: 235–238.
Sabio, E., Gonzólez-Martín, M. L., Ramiro, A. et al., Influence of the regeneration temperature on the phenols adsorption on activated carbon, Journal of Colloid and Interface Science, 2001, 242: 31–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bo, L., Chen, S., Quan, X. et al. Microwave assisted wet oxidation of p-nitrophenol. Sci. China Ser. E-Technol. Sci. 48, 220–232 (2005). https://doi.org/10.1360/03ye0604
Received:
Issue Date:
DOI: https://doi.org/10.1360/03ye0604