Abstract
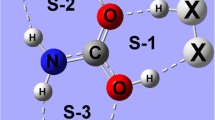
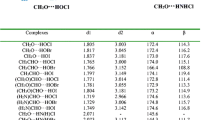
The nature of the intermolecular hydrogen bond for the furan-HCl and furan-CHCI3 complexes has been studied using ab initio calculations with MP2 level of theory. The new hydrogen bond type of C(CI)-H...O and π interactions are studied also. It is shown that, for the optimized geometries of furan-CHCI3, C-H bond lengths contract and vibrational frequencies are blue-shifted, while for the furan-HCl complex, H-CI bond lengths elongate and vibrational frequencies are red-shifted. In addition, the NBO analysis indicates that, for the furan-CHCI3 complex, the charge transfers from the lone pair of the proton acceptor to both σ *(CH) antibonding MO and lone pairs of CI atom.
Similar content being viewed by others
References
Steiner, T., Unrolling the hydrogen bond properties of CH...interactions, Chem. Commun., 1997: 727–734.
Rozas, I., Alkorta, I., Elguero, J., Bifurcated hydrogen bonds: Three-centered interactions, J. Phys. Chem., 1998, 102: 9925–9932.
Zhang, Y. H., Hao, J. K., Wang, X. et al., A theoretical study of some pseudo-π hydrogen-bonded complexes: cycopropane · HC1 and tetrahedrane · HC1, J. Mol. Struct.(Theochem.), 1998, 455: 85–99.
Li, Z. R., Li, Z. S., Huang, X. R. et al., Long range π-type hydrogen bond in the dimers (HF)2, (H2O)2, and H2O-HF, J. Phys. Chem., 2001, 105: 1163–1168.
Radu, C., James, E. J., Dihydrogen bonding: Structures, energetics, and dynamics, Chem. Rev., 2001, 101: 1963–1980.
Motdunori, T., Hidekazu, T., Koichiro, N. et al., Calorimetric and molecular orbital studies of hydrogen bonding between hydrogen fluoride and cyclic ethers, J. Am. Chem. Soc., 1978, 100: 7189–7196.
Shea, J. A., Kukolich, S. G., The rotational spectrum and molecular structure of the furan-HCl complex, J. Phys. Chem., 1986, 78(6): 3545–3551.
Mellouki, A., Lievin, J., Herman, M., The vibrational spectrum of pyrrole (C4H5N) and furan (C4H4O) in the gas phase, Chem. Phys., 2001, 271: 239–266.
Rozas, I., Alkorta, I., Elguero, J., Unusual hydrogen bonds: π interactions, J. Phys. Chem., 1997, 101: 9457–9463.
Jiang, J. C., Tsai, M. H., Ab initio study of the hydrogen bonding between pyrrole and hydrogen fluoride: A comparison of NH...F and FH...πinteractions, J. Phys. Chem., 1997, 101: 1982–1988.
Lopes, K. C., Pereira, F. S., Ramos, M. N. et al., An ab initio study of the C3H6-HX, C2H4-HX and C2H2-HX hydrogen-bonded complexes with X= F or Cl, Spectochimica Acta Part A, 2000, 57: 1339–1346.
Hobza, P., Havlas, Z., Blue-shifting hydrogen bonds, Chem. Rev., 2000, 100: 4253–4264.
Kryachko, E. S., Huyskens, T. Z., Theoretical study of the CH...0 interaction in fluoromethanes · H2O and chloromethanes · H2O complexes, J. Phys. Chem., 2001, 105: 7118–7125.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, L., Daiqian, X. & Guosen, Y. Theoretical study of the intermolecular hydrogen bond interaction for furan-HCl and furan-CHCl3 complexes. Sc. China Ser. B-Chem. 46, 113–118 (2003). https://doi.org/10.1360/03yb9016
Received:
Issue Date:
DOI: https://doi.org/10.1360/03yb9016