Abstract
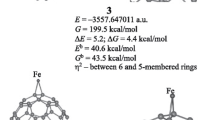
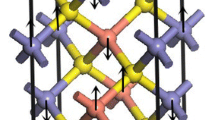
The electronic structure and chemical bonding in a recently synthesized inorganic fullerene-like molecule, [CuCl]20[Cp*FeP5]12[Cu-(CH3CN) + 2Cl−]5 has been studied by a density functional approach. Geometrical optimization of the three basic structural units of the molecule is performed with Amsterdam Density Functional Program. The results are in agreement with the experiment. Localized MO’s obtained by Boys-Foster method give a clear picture of the chemical bonding in this molecule. The reason why CuCl can react with Cp*FeP5 in solvent CH3CN to form the fullerene-like molecule is explained in terms of the soft-hard Lewis acid base theory and a new concept of covalence.
Similar content being viewed by others
References
Bai, J. F., Alexander, V. V., Manfred, S., Synthesis of inorganic fullerene-like molecules, Science, 2003, 300: 781–783.
Amsterdam Density Functional (ADF), Version 2003.2; Scientific Computing and Modeling, Theoretical Chemistry, Amsterdam: Virje University, 2003.
Vosko, S. H., Wilk, L., Nusair, M., Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis, Can. J. Phys., 1980, 58: 1200–1211.
Becke, A. D., Density-functional exchange-energy approximation with correct asymptotic behavior, Phys. Rev. A, 1988, 38: 3098- 3100.
Perdew, J. P., Density-functional approximation for the correlation energy of the inhomogeneous electron gas, Phys. Rev. B, 1986, 33: 8822–8824.
Boerrigter, P. M., Velde, G. T., Baerends, E. J., Three-dimensional numerical integration for electronic structure calculations, Int. J. Quant. Chem., 1988, 33: 87–113.
Velde, G. T., Baelends, E. J., Numerical integration for polyatomic systems, J. Comput. Phys., 1992, 99: 84–98.
Broyden, C. G., The convergence of a class of double-rank minimization algorithms 2, The new algorithm, J. of the Inst. for Math. and Applications, 1970, 6: 222–231.
Fletcher, R., A new approach to variable-metric algorithms, Computer Journal, 1970, 13: 317–322.
Goldfarb, D., A family of variable-metric algorithms derived by variational means, Math. Comp., 1970, 24: 23–26.
Shanno, D. F., Conditioning of quasi-Newton methods for function minimization, Mathematics of Computation, 1970, 24: 647–656.
Rüdenberg, C. K., Localized atomic and molecular orbitals, Rev. Mod. Phys., 1963, 35: 457–464.
Boys, S. F., Construction of some molecular orbitals to be approximately invariant for changes from one molecule to another, Rev. Mod. Phys., 1960, 32: 296–299.
Xu Guangxian, Structural rules of cluster compounds and related molecules (I)—The (nxcπ) formalism, Chemical J. Chinese Universities, 1984, 1: 100–112.
Xu Guangxian, Structural rules of cluster compounds and related molecules (II)—New definition of covalence, Molecular Science, 1983, 1(10): 1–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Xu, G. & Chen, Z. Study on the covalence of Cu and chemical bonding in an inorganic fullerene-like molecule, [CuCl]20[Cp*FeP5]12 [Cu-(CH3CN)+ 2Cl-]5, by a density functional approach. Sc. China Ser. B-Chem. 47, 91–97 (2004). https://doi.org/10.1360/03yb0177
Received:
Issue Date:
DOI: https://doi.org/10.1360/03yb0177