Abstract
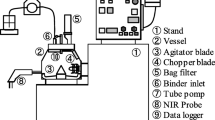
A circulating flow system consisting of a transparent U-bend flow loop, a mixing tank and a laser granulometer was set up for studying the kinetics hydrate formation and the pressure is up to 4 MPa. Refrigerant CCl2F2 (R12) hydrate formation experiments were performed using laser light scattering method at 277.1 K and pressures of 0.24 and 0.32 MPa. The liquid flow rates were in the range of 300–1400 L/h. The size distribution and density of R12 hydrate particles in pure water were measured using a laser granulometer. Experimental results show that the size of hydrate particles increases sharply at the initial stage and approaches gradually to a stable size. The hydrate particle concentration in the aqueous phase increases with pressure and circulating liquid flow rate. Based on the material balance, the mathematical model among gas consumption, average hydrate particle size and shading ratio has been established. The calculated results using the mathematical model accord well with the experimental gas consumption data.
Similar content being viewed by others
References
Vysniauskas, A., Bishnoi, P. R., Akinetic study of methane hydrate formation, Chem. Eng. Sci., 1983, 38(7): 1061–1072.
Englezos, P., Kalogerakis, N., Dholabhai, P. D. et al., Kinetics of formation of methane and ethane gas hydrates, Chem. Eng. Sci., 1987, 42(1): 2647–2658.
Englezos, P., Kalogerakis, N., Dholabhai, P. D. et al., Kinetics of gas hydrate formation from mixtures of methane and ethane, Chem. Eng. Sci., 1987, 42(1): 2659–2666.
Skovborg, P., Rasmussen, P., A mass transport limited model for the growth of methane and ethane gas hydrates, Chem. Eng. Sci., 1994, 49(8): 1131–1143.
Sloan, E. D., Clathrate Hydrates of Natural Gases, 2nd ed., New York: Marcel Dekker, 1998.
Nerheim, A. R., Svartaas, T. M., Samuelsen, E. K., Laser light scattering studies of gas hydrate formation kinetics, in Proc. 4th Intl. Offshore & Polar Eng. Conf. (ed. Chung, J. S.), Osaka: International Society of Offshore and Polar Engineers, 1994, 323–328.
Parent, J. S., Investigations into the nucleation behavior of the clathrate hydrates of natural gas components, MS Thesis, Univ. of Calgary, 1993.
Monfort, J. P., Nzihou, A., Light scattering kinetics study of cyclopropane hydrate growth, J. Crystal Growth, 1993, 128: 1182–1186.
Allen, T., Particle Size Measurement, 3rd ed. (Power Technology Series), London: Chapman & Hall, 1981.
Bylov, M., Rasmussen, P., Experimental determination of refractive index of gas hydrates, Chem. Eng. Sci., 1997, 52(19): 3295–3301.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, C., Chen, G. & Guo, T. R12 hydrate formation kinetics based on laser light scattering technique. Sc. China Ser. B-Chem. 46, 487–494 (2003). https://doi.org/10.1360/03yb0057
Received:
Issue Date:
DOI: https://doi.org/10.1360/03yb0057