Abstract
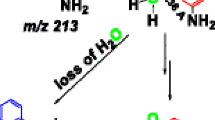
The cationized 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids were analyzed by the electrospray ionization tandem mass spectrometry (ESI-MS/MS). A rearrangement reaction leading to the C-terminal hydroxyl group transfer was observed. The sodium adducts of Fmoc-OH was formed. A possible rearrangement mechanism was proposed. The rearrangement reaction depended on the Fmoc group, metal ions and metal ion radius. It was shown that the Fmoc group has a strong affinity to the hydroxyl group in the gas phase.
Similar content being viewed by others
References
Chait, B. T., Wang, R., Kent, S. B. H. et al., Protein Ladder sequencing, Science, 1993, 262: 89.
Wilm, M., Shevchenko, A., Houthaeve, T. et al., Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry, Nature, 1996, 379: 466.
Sutton, C. W., Pemberton, K. S., Cottrell, J. S. et al., Identification of myocardial proteins from 2-dimensional gels by peptide mass fingerprinting, Electrophoresis, 1995, 16: 308.
Mann, M., Wilm, M., Error tolerant identification of peptides in sequence databases by peptide sequence tags, Anal. Chem., 1994, 66: 4390–4399.
Eng, J. K., Mccormack, A. L., Yates, J. R., An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database, J. Am. Soc. Mass Spectrom., 1994, 5: 976–989.
Weng, C. C., Peter, D. W., Fmoc Solid Phase Peptide Synthesis: A Practical Approach, Oxford: University Press, 2000, 1–8.
Chen, Z. Z., Chen, S. B., Li, Y. M. et al., Negative-ion ESI mass spectrometry of N-phosphoryl amino acids and dipeptides, Rapid Commun. Mass Spectrom., 2002, 16(8): 790–796.
Chen, Z. Z., Tong, Y. F., Li, Y. M. et al., Orientation of the peptide formation of N-phosphoryl amino acids in solution, Chinese Sci. Bull. (in Chinese), 2002, 47(13): 993–997.
Chen, J., Chen, Y., Zhao, Y. F. et al., Rearrangement of P-N to P-O bonds in mass spectra of N-diisopropyloxyphosphoryl amino acids/alcohols, Rapid Commun. Mass Spectrom., 2001, 15: 1936–1940.
Atherton, E., Sheppard, R. C., Solid Phase Peptide Synthesis: A Practical Approach, Oxford: IRL Press, 1989.
Wakamiya, T., Nishida, T., Togashi, R. et al., Preparations of NαFmoc-O-[(benzyloxy)hydroxyphosphinyl] β-hydroxy α-amino acid derivatives, Bull. Chem. Soc. Jpn., 1996, 69: 465–468.
Lynn, M. T., Ronald, C. O., Jeanette, A., Location of the alkali metal ion in gas-phase peptide complexes, J. Am. Chem. Soc., 1991, 113: 3668–3675.
Tang, X. J., Werner, E., Kenneth, G. S. et al., Daughter ion mass spectra from cationized molecules of small oligopeptides in a reflecting time-of-flight mass spectrometer, Anal. Chem., 1988, 60: 1791–1789.
Kevin, D. B., Simon, J. G., Intramolecular oxygen-18 isotopic exchange in the gas phase observed during the tandem mass spectrometric analysis of peptides, J. Am. Chem. Soc., 1992, 114: 64–71.
Richard, P. G., Ronald, L. C., Michael, L. G., Metal ion-peptide interactions in the gas phase: a tandem mass spectrometry study of alkali metal cationized peptides, J. Am. Chem. Soc., 1989, 111: 2835–2842.
Chen, J., Jiang, Y., Zhao, Y. F. et al., Rearrangement with formamide extrusion in the electrospray mass spectra of aminoacylbenzylamines, Rapid Commun. Mass Spectrom., 2001, 15: 1489–1493.
Nakagawa, M., Yamagaki, T., Nakanishi, H., Fluorescent modification for peptide sequencing by postsource decay-matrix assisted laser desorption/ionization-mass spectrometry, Electrophoresis, 2000, 21: 1651–1652.
Chen, J., Li, Y. M., Zhao, Y. F. et al., Novel phosphoryl derivatization method for peptide sequencing by electrospary ionization mass spectrometry, Rapid Commun. Mass Spectrom., 2002, 16: 531–536.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Du, J., Li, Y., Zhu, Z. et al. Rearrangement mechanism of the sodium adducts of Fmoc protected amino acids. Chin.Sci.Bull. 48, 2317–2319 (2003). https://doi.org/10.1360/03wb0093
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1360/03wb0093