Abstract
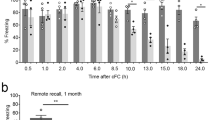
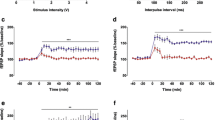
Activation of β-adrenoceptors in area CA1 of the hippocampus facilitates in vitro long-term potentiation (LTP) in this region. However, it is unclear if in vivo LTP in area CA1 and hippocampus-dependent learning are subjected to β-adrenergic regulation. To address this question, we investigated the effects of the β-adrenergic agonist L-isoproterenol or antagonist DL-propranolol on in vivo LTP of area CA1 and the spatial learning in Morris water maze. In the presence of L-isoproterenol (through local infusion into area CA1), the theta-pulse stimulation with the parameter of 10 Hz, 150 pulses/train, 1 train, a frequency weakly modifying synaptic strength, induced a robust LTP, and this effect was blocked when DL-propranolol was co-administered. By contrast, the theta-pulse stimulation with the parameter of 5 Hz, 150 pulses/train, 3 trains, a frequency strongly modifying synaptic strength, induced a significantly smaller LTP when DL-propranolol was administered into area CA1. Accordingly, DL-propranolol impaired the spatial learning in the water maze when infused into area CA1 20 min pretraining. Compared with control rats, the DL-propranolol-treated rats showed significantly slower learning in the water maze and subsequently exhibited poor memory retention at 24-h test. These results suggest that β-adrenoceptors in area CA1 are involved in regulating in vivo synaptic plasticity of this area and are important for spatial learning.
Similar content being viewed by others
References
Squire, L. R., Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans, Psychol. Rev., 1992, 99(2): 195–231.
Bliss, T. V., Collingridge, G. L. A., Synaptic model of memory: Long-term potentiation in the hippocampus, Nature, 1993, 361(6407): 31–39.
Eichenbaum, H., Otto, T., LTP and memory: Can we enhance the connection? TINS, 1993, 16(5): 163–164.
Malenka, R. C., Nicoll, R. A., Long-term potentiation-—A decade of progress? Science, 1999, 285(5435): 1870–1874.
Martin, S. J., Grimwood, P. D., Morris, R. G. M., Synaptic plasticity and memory: An evaluation of the hypothesis, Annu. Rev. Neurosci., 2000, 23: 649–711.
Morris, R. G. M., Synaptic plasticity and learning: Selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5, J. Neurosci., 1989, 9(9): 3040–3057.
Stevens, C. F. A., Million dollar question: Does LTP = memory? Neuron, 1998, 20(1): 1–2.
Brown, G. P., Blitzer, R. D., Connor, J. H. et al., Long-term potentiation induced by theta frequency stimulation is regulated by a protein phosphatase-1-operated gate, J. Neurosci., 2000, 20(21): 7880–7887.
Giovannini, M. G., Blitzer, R. D., Wong, T. et al., Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation, J. Neurosci., 2001, 21(18): 7053–7062.
Katsuki, H., Izumi, Y., Zorumski, C. F., Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region, J. Neurophysiol., 1997, 77(6): 3013–3020.
Thomas, M. J., Moody, T. D., Makhinson, M. et al., Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region, Neuron, 1996, 17(3): 475–482.
Winder, D. G., Martin, K. C., Muzzio, I. A. et al., ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors, Neuron, 1999, 24(3): 715–726.
Paxinos, G., Watson, C., The Rat Brain in Stereotaxic Coordinates, 2nd ed., San Diego: Academic Press, 1996.
Morris, R. G. M., Garrud, P., Rawlins, J. N. et al., Place navigation impaired in rats with hippocampal lesions, Nature, 1982, 297(5868): 681–683.
Morris, R. G. M., Anderson, E., Lynch, G. S. et al., Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5, Nature, 1986, 319(6056): 774–776.
Sandin, J., Nylander, I., Georgieva, J. et al., Hippocampal dynorphin B injections impair spatial learning in rats: A kappa-opioid receptor-mediated effect, Neuroscience, 1998, 85(2): 375–382.
McGaugh, J. L., Cahill, L., Roozendaal, B., Involvement of the amygdala in memory storage: Interaction with other brain systems, Proc. Natl. Acad. Sci. USA, 1996, 93(24): 13508–13514.
Ferry, B., Roozendaal, B., McGaugh, J. L., Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: A critical involvement of the amygdala, Biol. Psychiatry, 1999, 46(9): 1140–1152.
Abel, T., Nguyen, P. V., Barad, M. et al., Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory, Cell, 1997, 88(5): 615–626.
Blum, S., Moore, A. N., Adams, F. et al., A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory, J. Neurosci., 1999, 19(9): 3535–3544.
Schafe, G. E., Nadel, N. V., Sullivan, G. M. et al., Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP Kinase, Learn. Mem., 1999, 6(2): 97–110.
Selcher, J. C., Atikins, C. M., Trzaskos, J. M. et al., A necessity for MAP kinase activation in mammalian spatial learning, Learn. Mem., 1999, 6(5): 478–490.
Silva, A. J., Kogan, J. H., Frankland, P. W. et al., CREB and memory, Annu. Rev. Neurosci., 1998, 21: 127–148.
Impey, S., Obrietan, K., Storm, D. R., Making new connections: Role of ERK/MAP kinase signaling in neuronal plasticity, Neuron, 1999, 23(1): 11–14.
Kandel, E. R., The molecular biology of memory storage: A dialogue between genes and synapses, Science, 2001, 294(5544): 1030–1038.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ji, J., Zhang, X. & Li, B. β-adrenergic modulation of in vivo long-term potentiation in area CA1 and its role in spatial learning in rats. Sci. China Ser. C.-Life Sci. 46, 605–614 (2003). https://doi.org/10.1360/02yc0243
Received:
Issue Date:
DOI: https://doi.org/10.1360/02yc0243