Abstract
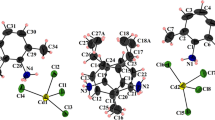
[Cd(NTO)4Cd(H2O)6] •4H2O was synthesized by mixing the aqueous solution of 3-nitro-1, 2,4-triazol-5-one (NTO) and cadmium carbonate. The single crystal structure was determined by a four-circle X-ray diffractometer. The crystal is monoclinic, space group C2/c with crystal parameters of a = 2.1229(3) nm, b = 0.6261(8) nm, = 2.1165(3) nm, β= 90.602 (3)°, V= 2.977(6) nm3, Z = 4, Dc = 2.055 g • cm-3, μ = 15.45 cm-1 and F(000) = 1824. 2523 observable independent reflections with F04σ(F0) were used for the determination and refinement of the crystal structure. Lorentz-polarization and absorption correction were applied. The final R is 0.0282 and wR = 0.0792. The analytical results show that the Cd+2 has two kinds of coordinate bonds in one crystal. One Cd+2 coordinates with 4 NTO anions and another coordinates with 6 water molecules to form a binucleate complex with a structure of tetrahedron and tetragonal bipyramid, respectively. By using SCF-PM3-MO method, the electron structure of cadmium complex of NTO has been calculated. The analysis of the calculated results shows that when [Cd(NTO)4Cd(H2O)6] • 4H2O is heated, the crystallization waters will be dissociated first and the ligand waters second and NO2 group has priority of leaving when NTO− is decomposed. Analysis of the energy level and composition of localized molecular orbitals indicates that both the two Cd2+ bond to the coordinating atom with 5s
Similar content being viewed by others
References
Lee, Y., Chapman, L. Coburn, M. D., A less sensitive explosive, 3-nitro-l,2,4-triazol-5-one, Journal of Energetic Materials, 1987, (5): 27–31.
Lee, Y., Coburn, M. D., 3-nitro-l,2,4-triazol-5-one, a less sensitive explosive, US Patent 4 733 610, 1988.
Agrawal, J. P., Walley, S. M., Field, J. E., A high-speed photographie study of the impact initiation of hexanitro-hexaaza-isowurtzitane and nitrotriazolone, Combustion and Flame, 1998, 112: 62–72.
Zhang, T. L., Hu, R. Z., Li, F. P. et al., Preparation and crystal structure of [Mn(H2O)6](NTO)2 · 2H2O, Energetic Materials (in Chinese), 1993, 1(1): 37–42.
Zhang, T. L., Hu, R. Z., Li, F. P. et al., Preparation, molecular structure and thermal decomposition mechanism of [Cu(NTO)2 (H2O)2] · 2H2O, Chinese Science Bulletin, 1993, 38(16): 1350–1353.
Song, J. R., Hu, R. Z., Li, F. P. et al., Preparation, crystal structure and thermal decomposition mechanism of [Co(H2O)6](NTO)2 · 2H2O, Chinese Science Bulletin, 1996, 41(21): 1806–1810.
Song, J. R., Hu, R. Z., Li, F. P. et al., Preparation, crystal structure and thermal decomposition mechanism of [Ni(H2O)6](NTO)2· 2H2O, Journal of Northwest University (Natural Science Edition) (in Chinese), 1997, 27(1): 29–33.
Chen, Z. X., Xiao, H. M., Song, J. R. et al., Quantum-chemical study on complex [Cu(NTO)2 (H2O)2] · 2H2O, Journal of Inorganic Chemistry (in Chinese), 1998, 14(1): 92–95.
Chen, Z. X., Xiao, H. M., Song, J. R. et al., Molecular orbital studies on the complexes of Mn, Co and Ni with NTO(3-nitro-l,2,4-triazol-5-one), Journal of Molecular Science (in Chinese), 1997, 13(1): 31–36.
Stewart, J. J. P., Optimization of parameters for semiempirical method, J. Comput, 1989, 10(2): 209–220.
Dean, J. A., Lange's Handbook of Chemistry, 13th ed., New York: McGraw-Hill Book Company, 1985, 3–122.
Song, J. R., Hu, R. Z., Kang, B. et al., Thermal decomposition mechanism and thermodynamic properties of [Cd(NTO)4Cd(H2O)6] · 4H2O, Journal of Thermal Analysis and Calorimetry, 1999, 55: 797–806.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, J., Ma, H., Huang, J. et al. Crystal structure and quantum chemical investigation of [Cd(NTO)4Cd(H2O)6] · 4H2O. Sc. China Ser. B-Chem. 46, 302–312 (2003). https://doi.org/10.1360/02yb0184
Received:
Issue Date:
DOI: https://doi.org/10.1360/02yb0184