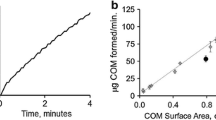
Abstract
Crystallization of calcium oxalate is studied mainly in the diluted healthy urine using scanning electron microscopy (SEM), and is compared with the crystallization in the diluted pathological urine. It suggests that the average sizes of calcium oxalate crystals are not in direct proportion to the concentrations of Ca2+ and Ox2- ions. Only in the concentration range of 0.60-0.90 mmol/L can larger size of CaOx crystals appear. When the concentrations of Ca2+ and Ox2- ions are 1.20, 0.80, 0.60, 0.30 and 0.15 mmol/L in the healthy urine, the average sizes of calcium oxalate crystallites are 9.5 X 6.5, 20.0 X 13.5 and 15.0 jj,m X 10.0 jj,m, respectively, for the former three samples after 6 d crystallization. No crystal appears even after 30 d crystallization for the samples of concentrations of 0.30 and 0.15 mmol/L due to their low supersaturations. The results theoretically explain why the probability of stone forming is clinically not in direct proportion to the concentrations of Ca2+ and Ox2- ions. Laser scattering technology also confirms this point. The reason why healthy human has no risk of urinary stone but stone-formers have is that there are more urinary macromolecules in healthy human urines than that in stone-forming urines. These macromolecules may control the transformation in CaOx crystal structure from monohydrate cal-cium oxalate (COM) to dihydrate calcium oxalate (COD). COD has a weaker affinity for renal tubule cell membranes than COM. No remarkable effect of the crystallization time is observed on the crystal morphology of CaOx. All the crystals are obtuse hexagon. However, the sizes and the number of CaOx crystals can be affected by the crystallization time. In the early stage of crystalli-zation (1-6 d), the sizes of CaOx crystals increase and the number of crystal particles changes little as increasing the crystallization time due to growth control. In the middle and late stages (6-30 d), the number of crystals increases markedly while the growth rate changes little due to the nucleation control.
Similar content being viewed by others
References
Ouyang, J. M., Chemical basis in the investigation of calcium oxalate stones, Chem. Bull, (in Chinese), 2002, (5): 326- 333.
Mustafi, D., Nakagawa, Y., Makinen, M. W., ENDOR studies of VO2+: probing protein-metal ion interactions in nephrocalcin, Cell Mol. Biol. (Noisy-le-grand), 2000, 46 (8): 1345–1360.
Buchholz, N. P., Kim, D. S., Grover, P. K. et al., The effect of warfarin therapy on the charge properties of urinary prothrombin fragment 1 and crystallization of calcium oxalate in undiluted human urine, J. Bone Miner. Res., 1999, 14(6): 1003–1012.
Tunik, L., Garti, N., Morphological and phase changes in calcium oxalate crystals growth in the presence of sodium diisooctal sulfosuccinate, J. Cryst. Growth, 1996, 167: 748–755.
Messa, P., Marangella, M., Paganin, L. et al., Different dietary calcium intake and relative supersaturation of calcium oxalate in the urine of patients forming renal stones, Clin. Sci., 1997, 93: 257–263.
Nenow, D., Vitkov, L., Effect of the opposite directions on the crystal face upon the growth kinetics of weddellite, J. Cryst. Growth, 1997, 182: 461–464.
Laurence, M. E., Levillain, P., Lacour, B. et al., Advantage of zero-crossing-point first-derivative spectrophotometry for the quantification of calcium oxalate crystalline phases by infrared spectrophotometry, Clin. Chim. Acta, 2000, 298: 1- 11.
Huang, S. T., Solid X-ray Study (in Chinese), Beijing: Higher Education Press, 1985, 215–218.
Liu, G D., Liu, X., Food therapy in urolithiasis, Chin. J. Urol. (in Chinese), 1999, 14(7): 277–279.
Tai, Z. H., Bioinorganic solid-state chemistry, J. Inorg. Chem. (in Chinese), 1994, 10 (3): 330–337.
Wu Han University, Analytical Chemistry (in Chinese), Beijing: Higher Education Press, 1982, 415–416.
Khan, S. R., Whalen, P. O., Glenton, P. A., Heterogeneous nucleation of calcium oxalate crystals in the presence of membrane vesicles, J. Cryst. Growth, 1993, 134: 211–218.
Jiang, X. J., Feng, T., Mi, P. et al., Expression of osteopontin mRNA in normal rata kidney, Chin. J. Urol. (in Chinese), 1998,19(4): 199–201.
Dai, Y. D., Shen, J. Y, Mechanism of bio-mineralization, Chin. J. Zoology (in Chinese), 1995, 30 (5): 55–58.
Falini, G, Albeck, S., Akkaki, L., Control of aragonite or calcite polymorphism by mollusk shell macromolecules, Science, 1996,271:67–69.
Wesson, A. J., Worcester, M. E., Wiessner, J. H. et al., Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules, Kidney Int., 1998, 53: 952–957.
Mandel, N., Crystal-membrane interaction in kidney stone disease, J. Am. Soc. Nephrol 5 (Supp. 1), 1994: S37-S45.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouyang, J., Yao, X., Su, Z. et al. Simulation of calcium oxalate stone in vitro . Sc. China Ser. B-Chem. 46, 234–242 (2003). https://doi.org/10.1360/02yb0033
Received:
Issue Date:
DOI: https://doi.org/10.1360/02yb0033