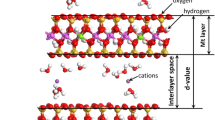
Abstract
Interlayer swelling of hydrated montmorillonite is an important issue in clay mineralogy. Although the swelling behavior of montmorillonite under ambient conditions has been investigated comprehensively, the effects of basin conditions on the hydration and swelling behaviors of montmorillonite have not been characterized thoroughly. In the present study, molecular dynamics simulations were employed to reveal the swelling behavior and changes in the interlayer structure of Namontmorillonite under the high temperatures and pressures of basin conditions. According to the calculation of the immersion energy, the monolayer hydrate becomes more stable than the bilayer hydrate at a burial depth of 7 km (at a temperature of 518 K and a lithostatic pressure of 1.04 kbar). With increasing burial depth, the basal spacings of the monolayer and bilayer hydrates change to varying degrees. The density-distribution profiles of interlayer species exhibit variation in the hydrate structures due to temperature and pressure change, especially in the structures of bilayer hydrate. With increasing depth, more Na+ ions prefer to distribute closer to the clay layers. The mobility of interlayer water and ions increases with increasing temperature, while increasing pressure caused the mobility of these ions to decrease.
Similar content being viewed by others
References
Anderson, R., Ratcliffe, I., Greenwell, H., Williams, P., Cliffe, S., and Coveney, P. (2010) Clay swelling — a challenge in the oilfield. Earth-Science Reviews, 98, 201–216.
Berend, I., Cases, J.M., Francois, M., Uriot, J.P., Michot, L., Masion, A., and Thomas, F. (1995) Mechanism of adsorption and desorption of water-vapor by homoionic montmorillonites. 2. The Li+, Na+, K+, Rb+ and Cs+-exchanged forms. Clays and Clay Minerals, 43, 324–336.
Boek, E.S. and Sprik, M. (2003) Ab initio molecular dynamics study of the hydration of a sodium smectite clay. Journal of Physical Chemistry B, 107, 3251–3256.
Boek, E.S., Coveney, P.V., and Skipper, N.T. (1995a) Molecular modeling of clay hydration: A study of hysteresis loops in the swelling curves of sodium montmorillonites. Langmuir, 11, 4629–4631.
Boek, E.S., Coveney, P.V., and Skipper, N.T. (1995b) Monte Carlo molecular modeling studies of hydrated Li-, Na-, and K-smectites: Understanding the role of potassium as a clay swelling inhibitor. Journal of the American Chemical Society, 117, 12608–12617.
Bourg, I.C. and Sposito, G. (2010) Connecting the molecular scale to the continuum scale for diffusion processes in smectite-rich porous media. Environmental Science & Technology, 44, 2085–2091.
Brown, G. and Brindley, G.W. (1980) Crystal Structures of Clay Minerals and their X-ray Identification. Monograph 5, Mineralogical Society of Great Britian & Ireland.
Cases, J.M., Berend, I., Besson, G., Francois, M., Uriot, J.P., Thomas, F., and Poirier, J.E. (1992) Mechanism of adsorption and desorption of water-vapor by homoionic montmorillonite. 1. The sodium-exchanged form. Langmuir, 8, 2730–2739.
Cases, J.M., Berend, I., Francois, M., Uriot, J.P., Michot, L.J., and Thomas, F. (1997) Mechanism of adsorption and desorption of water vapor by homoionic montmorillonite. 3. The Mg2+, Ca2+, Sr2+ and Ba2+ exchanged forms. Clays and Clay Minerals, 45, 8–22.
Cygan, R.T., Liang, J.-J., and Kalinichev, A.G. (2004) Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. The Journal of Physical Chemistry B, 108, 1255–1266.
Cygan, R.T., Greathouse, J.A., Heinz, H., and Kalinichev, A.G. (2009) Molecular models and simulations of layered materials. Journal of Materials Chemistry, 19, 2470–2481.
Dazas, B., Ferrage, E., Delville, A., and Lanson, B. (2014) Interlayer structure model of tri-hydrated low-charge smectite by X-ray diffraction and Monte Carlo modeling in the Grand Canonical ensemble. American Mineralogist, 99, 1724–1735.
De Pablo, L., Chavez, M.L., and De Pablo, J.J. (2005) Stability of Na-, K-, and Ca-montmorillonite at high temperatures and pressures: A Monte Carlo simulation. Langmuir, 21, 10874–10884.
De Siqueira, A.V.C., Skipper, N.T., Coveney, P.V., and Boek, E.S. (1997) Computer simulation evidence for enthalpy driven dehydration of smectite clays at elevated pressures and temperatures. Molecular Physics, 92, 1–6.
De Siqueira, A.V., Lobban, C., Skipper, N.T., Williams, G.D., Soper, A.K., Done, R., Dreyer, J.W., Humphreys, R.J., and Bones, J.A. (1999) The structure of pore fluids in swelling clays at elevated pressures and temperatures. Journal of Physics: Condensed Matter, 11, 9179.
Deming, D. (2002) Introduction to Hydrogeology. McGraw-Hill, New York.
Ferrage, E., Lanson, B., Malikova, N., Plançon, A., Sakharov, B.A., and Drits, V.A. (2005a) New insights on the distribution of interlayer water in bi-hydrated smectite from X-ray diffraction profile modeling of 00l reflections. Chemistry of Materials, 17, 3499–3512.
Ferrage, E., Lanson, B., Sakharov, B.A., and Drits, V.A. (2005b) Investigation of smectite hydration properties by modeling experimental X-ray diffraction patterns: Part I. montmoril lonite hydration properties. American Mineralogist, 90, 1358–1374.
Ferrage, E., Lanson, B., Michot, L.J., and Robert, J.-L. (2010) Hydration properties and interlayer organization of water and ions in synthetic Na-smectite with tetrahedral layer charge. Part 1. Results from X-ray diffraction profile modeling. Journal of Physical Chemistry C, 114, 4515–4526.
Greathouse, J.A., Stellalevinsohn, H.R., Denecke, M.A., Bauer, A., and Pabalan, R.T. (2005) Uranyl surface complexes in a mixed-charge montmorillonite: Monte Carlo computer simulation and polarized XAFS results. Clays and Clay Minerals, 53, 278–286.
Guggenheim, S. and Van Groos, A.K. (2001) Baseline studies of The Clay Minerals Society source clays: thermal analysis. Clays and Clay Minerals, 49, 433–443.
Heinz, H., Koerner, H., Anderson, K.L., Vaia, R.A., and Farmer, B. (2005) Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chemistry of Materials, 17, 5658–5669.
Hensen, E.J. and Smit, B. (2002) Why clays swell? The Journal of Physical Chemistry B, 106, 12664–12667.
Holmboe, M. and Bourg, I.C. (2014) Molecular dynamics simulations of water and sodium diffusion in smectite interlayer nanopores as a function of pore size and temperature. The Journal of Physical Chemistry C, 118, 1001–1013.
Holmboe, M., Wold, S., and Jonsson, M. (2012) Porosity investigation of compacted bentonite using XRD profile modeling. Journal of Contaminant Hydrology, 128, 19–32.
Huang, W.L., Bassett, W.A., and Wu, T.C. (1994) Dehydration and hydration of montmorillonite at elevated temperatures and pressures monitored using synchrotron radiation. American Mineralogist, 79, 683–691.
Hunt, J.M. (1990) Generation and migration of petroleum from abnormally pressured fluid compartments. AAPG Bulletin, 74, 1–12.
Kim, N., Kim, Y., Tsotsis, T.T., and Sahimi, M. (2005) Atomistic simulation of nanoporous layered double hydroxide materials and their properties. I. Structural modeling. Journal of Chemical Physics, 122.
Kumar, P.P., Kalinichev, A.G., and Kirkpatrick, R.J. (2007) Molecular dynamics simulation of the energetics and structure of layered double hydroxides intercalated with carboxylic acids. Journal of Physical Chemistry C, 111, 13517–13523.
Laird, D.A. (1999) Layer charge influences on the hydration of expandable 2:1 phyllosilicates. Clays and Clay Minerals, 47, 630–636.
Liu, X.D. and Lu, X.C. (2006) A thermodynamic understanding of clay-swelling inhibition by potassium ions. Angewandte Chemie, International Edition, 45, 6300–6303.
Liu, X.D., Lu, X.C., Wang, R.C., and Zhou, H.Q. (2008) Effects of layer-charge distribution on the thermodynamic and microscopic properties of Cs-smectite. Geochimica et Cosmochimica Acta, 72, 1837–1847.
Loewenstein, W. (1954) The distribution of aluminum in the tetrahedra of silicates and aluminates. American Mineralogist, 39, 92–96.
Meunier, A. (2005) Clays. Springer Science & Business Media.
Odriozola, G. and Guevara-Rodríguez, F.d.J. (2004) Namontmorillonite hydrates under basin conditions: Hybrid Monte Carlo and molecular dynamics simulations. Langmuir, 20, 2010–2016.
Osakai, T., Tokura, A., Ogawa, H., Hotta, H., Kawakami, M. and Akasaka, K. (2003) Temperature effect on the selective hydration of sodium ion in nitrobenzene. Analytical Sciences, 19, 1375–1380.
Petit, S. and Madejová, J. (2013) Fourier transform infrared spectroscopy. Pp. 213–231 in: Handbook of Clay Science, 2nd edition (F. Bergaya and G. Lagaly, editors). Developments in Clay Science, 5, Elsevier, Amsterdam.
Plimpton, S. (1995) Fast parallel algorithms for short-range molecular dynamics. Journal of Computational Physics, 117, 1–19.
Rick, S.W., Stuart, S.J., and Berne, B.J. (1994) Dynamical fluctuating charge force-fields - application to liquid water. Journal of Chemical Physics, 101, 6141–6156.
Shahriyari, R., Khosravi, A., and Ahmadzadeh, A. (2013) Nanoscale simulation of Na-montmorillonite hydrate under basin conditions, application of CLAYFF force field in parallel GCMC. Molecular Physics, 111, 3156–3167.
Smith, D.E. (1998) Molecular computer simulations of the swelling properties and interlayer structure of cesium montmorillonite. Langmuir, 14, 5959–5967.
Smith, D.E., Wang, Y., Chaturvedi, A., and Whitley, H.D. (2006) Molecular simulations of the pressure, temperature, and chemical potential dependencies of clay swelling. The Journal of Physical Chemistry B, 110, 20046–20054.
Tambach, T.J., Hensen, E.J., and Smit, B. (2004) Molecular simulations of swelling clay minerals. The Journal of Physical Chemistry B, 108, 7586–7596.
Teich-McGoldrick, S.L., Greathouse, J.A., Jové-Colón, C.F., and Cygan, R.T. (2015) Swelling properties of montmorillonite and beidellite clay minerals from molecular simulation: comparison of temperature, interlayer cation, and charge location effects. The Journal of Physical Chemistry C, 119, 20880–20891.
Teppen, B.J., Rasmussen, K., Bertsch, P.M., Miller, D.M., and Schäfer, L. (1997) Molecular dynamics modeling of clay minerals. 1. Gibbsite, kaolinite, pyrophyllite, and beidellite. The Journal of Physical Chemistry B, 101, 1579–1587.
Wang, J., Kalinichev, A.G., and Kirkpatrick, R.J. (2006) Effects of substrate structure and composition on the structure, dynamics, and energetics of water at mineral surfaces: A molecular dynamics modeling study. Geochimica et Cosmochimica Acta, 70, 562–582.
Wang, J.W., Kalinichev, A.G., and Kirkpatrick, R.J. (2004) Molecular modeling of water structure in nano-pores between brucite (001) surfaces. Geochimica et Cosmochimica Acta, 68, 3351–3365.
Wu, T.C., Bassett, W.A., Huang, W.L., Guggenheim, S., and Koster van Groos, A.F. (1997) Montmorillonite under high H2O pressures: Stability of hydrate phases, rehydration hysteresis, and the effect of interlayer cations. American Mineralogist, 82, 69–78.
Xie, X., Bethke, C.M., Li, S., Liu, X., and Zheng, H. (2001) Overpressure and petroleum generation and accumulation in the Dongying Depression of the Bohaiwan Basin, China. Geofluids, 1, 257–271.
Xu, W.Z., Johnston, C.T., Parker, P., and Agnew, S.F. (2000) Infrared study of water sorption on Na-, Li-, Ca-, and Mg-exchanged (SWy-1 and SAz-1) montmorillonite. Clays and Clay Minerals, 48, 120–131.
Zhang, L.H., Lu, X.C., Liu, X.D., Zhou, J.H., and Zhou, H.Q. (2014) Hydration and mobility of interlayer ions of (Nax, Cay)-montmorillonite: A molecular dynamics study. The Journal of Physical Chemistry C, 118, 29811–29821.
Zheng, Y. and Zaoui, A. (2013) Temperature effects on the diffusion of water and monovalent counterions in the hydrated montmorillonite. Physica A: Statistical Mechanics and Its Applications, 392, 5994–6001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, J., Lu, X. & Boek, E.S. Changes in the Interlayer Structure and Thermodynamics of Hydrated Montmorillonite Under Basin Conditions: Molecular Simulation Approaches. Clays Clay Miner. 64, 503–511 (2016). https://doi.org/10.1346/CCMN.2016.0640412
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2016.0640412