Abstract
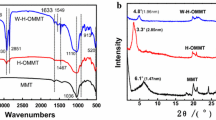
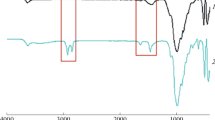
The main objective of the present work was to functionalize nanoclays with organosilanes and surfactant in order to facilitate the dispersion of the nanofillers in the host fluoroelastomer (FKM) polymer matrix. Better dispersion was achieved by improving interaction between the clay polymer nanocomposite (CPN) constituents. The first part of this study investigated modification of montmorillonite (Mnt) using different saturated and unsaturated alkyl silanes and an alkyl hydrocarbon ammonium quaternary surfactant. Silicon magic angle spinning nuclear magnetic resonance spectroscopy, thermal gravimetric analysis (TGA), elemental analysis, X-ray diffraction (XRD), and Fourier transform infrared spectroscopy were used to characterize the silane-grafted clays. Results indicated that the amount of silane grafted depended on the specific structure of the silane. Silane-grafted Mnt was also modified with ionic surfactants intercalated between the clay layers. A 169% increase in the clay basal spacing (from initial spacing of 10.0 Å to 26.9 Å) was achieved. The second part of the study successfully synthesized FKM nanocomposites containing custom-functionalized Mnt, with the aim of producing reinforced high-performance materials. The effects of clay modification on the morphology and thermal properties of the CPN were studied using XRD, TGA, scanning electron microscopy, and transmission electron microscopy. The CPN made with the modified clay exhibited greater thermal stability than the CPN of the commercially available modified Mnt, with a degradation onset point ~ 40°C higher.
Similar content being viewed by others
References
Abbehausen, C., Formiga, A.L.B., Sabadini, E., and Yoshid, I.V.P. (2010) A β-cyclodextrin/siloxane hybrid polymer: synthesis, characterization and inclusion complexes. Journal of Brazilian Chemical Society, 21, 1867–1876.
Akelah, A. and Moet, A. (1996) Polymer-clay nanocomposites: Free-radical grafting of polystyrene on to organophilic montmorillonite interlayers. Journal of Materials Science, 31, 3589–3596.
Al-haj Ali, M. and Elleithy, R.H. (2011) Viscoelastic properties of polypropylene/organo-clay nano-composites prepared using miniature lab mixing extruder from masterbatch. Journal of Applied Polymer Science, 121, 27–36.
Ameduri, B., Boutevin, B., and Kostov, G. (2001) Fluoroelastomers: synthesis, properties and applications. Progress in Polymer Science, 26, 105–187.
Bergaya, F. and Lagaly, G. (2001) Surface modification of clay minerals. Applied Clay Science, 19, 1–3.
Bergaya, F., Jaber, M., and Lambert, J.F. (2011) Clays and clay minerals. Pp. 1–44 in: Rubber-Clay Nanocomposites, Science, Technology and Applications (M. Galimberti, editor). Wiley, Hoboken, New Jersey.
Bergaya, F., Detellier, C., Lambert, J.-F., and Lagaly, G. (2013) Introduction to clay polymer nanocomposites. Pp. 655–678 in: Handbook of Clay Science, 2nd edition. Developments in Clay Science, Volume 5, Elsevier, Amsterdam.
Borse, N.K. and Kamal, M.R. (2006) Melt processing effects on the structure and mechanical properties of PA-6/clay nanocomposites. Polymer Engineering and Science, 46, 1094–1103.
Bukka, K. and Miller, J.D. (1992) FTIR study of deuterated montmorillonites: Structural features relevant to pillared clay stability. Clays and Clay Minerals, 40, 92–102.
Chen, G.X., Choi, J.B., and Yoon, J.S. (2005) The role of functional group on the exfoliation of clay in poly(L-lactide). Macromolecular Rapid Communication, 26, 183–187
Coates, J. (2000) Interpretation of infrared spectra, a practical approach. Encyclopedia of Analytical Chemistry, 10815–10837.
Daniel, L.M., Frost, R.L., and Zhu, H.Y. (2008) Edge-modification of Laponite with dimethyl-octylmethoxysilane. Journal of Colloid Interface Science, 321, 302–309.
Das, A., Stöckelhuber, K.W., Jurk, R., Jehnichen, D., and Heinrich, G. (2011) A general approach to rubber—montmorillonite nanocomposites: Intercalation of stearic acid. Applied Clay Science, 51, 117–125.
Dennis, H.R., Hunter, D.L., Chang, D., White, J.L., Cho, J.W., and Paul, D.R. (2001) Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer, 42, 9513–9522.
Dong, J., Ozaki, Y., and Nakashima, K. (1997) Infrared, Raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules, 30, 1111–1117.
El Rassy, H. and Pierre, A.C. (2005) NMR and IR spectroscopy of silica aerogels with different hydrophobic characteristics. Journal of Non-Crystalline Solids, 351, 1603–1610.
He, H., Frost, L.R., and Zhu, J. (2004) Infrared study of HDTMA+ intercalated montmorillonite. Spectrochimica Acta Part A, 60, 2853–2859.
He, H., Duchet, J., Galy, J., and Gerard, J.F. (2005) Grafting of swelling clay materials with 3-aminopropyltriethoxysilane. Journal of Colloid and Interface Science, 288, 171–176.
Heinz, H. (2012) Clay minerals for nanocomposites and biotechnology: surface modification, dynamics and responses to stimuli. Clay Minerals, 47, 205–230.
Hermosin, M.C. and Cornejo, J. (1986) Methylation of sepiolite and palygorskite with diazomethane. Clays and Clay Minerals, 34, 591–596.
Herrera, N.N., Letoffe, J.M., Putaux, J.L., David, L., and Bourgeat-Lami, E. (2004) Aqueous dispersions of silane-functionalized Laponite clay platelets. A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir, 20, 1564–1571.
Hrachová, J., Komadel, P., and Chodák, I. (2008) Effect of montmorillonite modification on mechanical properties of vulcanized natural rubber composites. Journal of Materials Science, 43, 2012–2017.
Hussain, F., Hojjati, M., Okamoto, M., and Gorga, R.E. (2006) Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. Journal of Composite Materials, 43, 3107–3123.
Huskic, M., Zigon, M., and Ivanković, M. (2013) Comparison of the properties of clay polymer nanocomposites prepared by montmorillonite modified by silane and by quaternary ammonium salts. Applied Clay Science, 85, 109–115.
Joo, J.H., Shim, J.H., Choi, J.H., Choi, C.H., Kim, D.S., and Yoon, J.S. (2008) Effect of the silane modification of an organoclay on the properties of polypropylene/clay composites. Journal of Applied Polymer Science, 109, 3645–3650.
Lakshminarayanan, S., Lin, B., and Sundararaj, U. (2009) Effect of clay surfactant type and clay content on the rheology and morphology of uncured fluoroelastomer/clay nanocomposites prepared by melt-mixing. Journal of Applied Polymer Science, 112, 3597–3604.
Lambert, J.-F. and Bergaya, F. (2013) Smectite-polymer nanocomposites. Pp. 670–706 in: Handbook of Clay Science, 2nd edition. Developments in Clay Science, Volume 5, Elsevier, Amsterdam.
Marynick, D.S. and Dixon, D.A. (1977) Electron affinity of the methyl radical: Structures of CH3 and CH −3 . Proceedings of the National Academy of Sciences of the United States of America, 74, 410–413.
Mingliang, G. and Demin, J. (2008) Influence of organoclay prepared by solid state method on the morphology and properties of polyvinyl chloride/organoclay nanocomposites. Journal of Elastomers and Plastics, 40, 223–235.
Mittal, V., Kim, J.K., and Pal, K. (2011) Recent Advances in Elastomeric Nanocomposites. Springer-Verlag, Berlin, Heidelberg.
Modesti, M., Lorenzetti, A., Bon, D., and Besco, S. (2005) Effect of processing conditions on morphology and mechanical properties of compatibilized polypropylene nanocomposite. Polymer, 46, 10237–10245.
Monasterio, F.E. (2010) Effect of the organic groups of difunctional silanes on the preparation of coated clays for olefin polymer modification. Clay Minerals, 45, 489–502.
Morrison, R.T. and Boyd, R.N. (1983) Organic Chemistry, 4th edition. New York University, New York.
Norrish, K. (1954) The Swelling of Montmorillonite. Division of Soils, C.S.I.R.O., Adelaide, Australia.
Orprecio, R. and Evans, C.H. (2003) Polymer-immobilized cyclodextrin trapping of model organic pollutants in flowing water streams. Journal of Applied Polymer Science, 90, 2103–2110.
Park, M., Shim, I.K., Jung, E.Y., and Choy, J.H. (2004) Modification of external surface of Laponite by silane grafting. Journal of Physical Chemistry of Solids, 65, 499–501.
Paul, D.R. and Robeson, L.M. (2008) Polymer nanotechnology: Nanocomposites. Polymer, 49, 3187–3204.
Pramanik, S., Das, G., and Karak, N. (2013) Facile preparation of polyaniline nanofibers modified bentonite nanohybrid for gas sensor application. The Royal Society of Chemistry Advances, 3, 4574–4581.
Qian, Z., Zhou, H., Xu, X., Ding, Y., Zhang, S., and Yang, M. (2009) Effect of the grafted silane on the dispersion and orientation of clay in polyethylene nanocomposites. Polymer Composite, 30, 1234–1242.
Shanmugharaj, A.M., Rhee, K.Y., and Ryu, S.H. (2006) Influence of dispersing medium on grafting of aminopropyltriethoxysilane in swelling clay materials. Journal of Colloid and Interface Science, 298, 854–859.
Shen, W., He, H., Zhu, J., Yuan, P., and Frost, R.L. (2007) Grafting of montmorillonite with different functional silanes via two different reaction systems. Journal of Colloid and Interface Science, 313, 268–273.
Stuart, B.H. (2004) Infrared Spectroscopy: Fundamentals and Applications. Wiley, New Jersey.
Smidt, E., Bhm, K., and Schwanninger, M. (2011) The application of FT-IR spectroscopy in waste management. Pp. 405–430 in: Fourier Transforms — New Analytical Approaches and FTIR Strategies (G. Nikolic, editor). InTech, Rijeka, Croatia.
Tian, R., Sitez, O., Li, M., Hu, W., Chabal, Y.J., and Gao, J. (2010) Infrared characterization of interfacial Si-O bond formation on silanized flat SiO2/Si surfaces. Langmuir, 26, 4563–4566.
Valsecchi, R., Torlaj, L., Turri, S., Tonelli, C., and Levi, M. (2011) Barrier properties in hydrogenated acrylonitrile butadiene rubber compounds containing organoclays and perfluoropolyether additives. Journal of Applied Polymer Science, 119, 3476–3482.
Wang, Y., Wang, X., Duan, Y., Liu, Y., and Du, S. (2011) Modification of montmorillonite with poly(oxypropylene) amine hydrochlorides: Basal spacing, amount intercalated, and thermal stability. Clays and Clay Minerals, 59, 507–517.
Wu, Y.P., Jia, Q.X., Yu, D.S., and Zhang, L.Q. (2004) Modeling Young’s modulus of rubber—clay nanocomposites using composite theories. Polymer Testing, 23, 903–909.
Xie, W., Gao, Z., Pan, W.P., Hunter, D., Singh, A., and Vaia, R. (2001) Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chemistry of Materials, 13, 2979–2990.
Xu, X., Ding, Y., Wang, F., Wen, B., Zhang, J., Zhang, S., and Yang, M. (2009) Effects of silane grafting on the morphology and thermal stability of poly(ethylene terephthalate)/clay nanocomposites. Polymer Composite, 31, 825–834.
Zhu, L. and Xanthos, M. (2004) Effects of process conditions and mixing protocols on structure of extruded polypropylene nanocomposites. Journal of Applied Polymer Science, 93, 1891–1899.
Zumdahl, S.S. (1999) Chemistry. 5th edition. Houghton Mifflin Harcourt, Boston, Massachusetts, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khajehpour, M., Gelves, G.A. & Sundararaj, U. Modification of Montmorillonite with Alkyl Silanes and Fluorosurfactant for Clay/fluoroelastomer (FKM) Nanocomposites. Clays Clay Miner. 63, 1–14 (2015). https://doi.org/10.1346/CCMN.2015.0630101
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2015.0630101