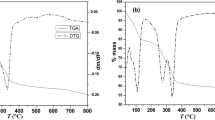
Abstract
Layered double hydroxides (LDH) are extremely important materials for industrial processes and in the environment, and their physical-chemical behavior depends in large part on their hydration state, but the characterization of these hydration effects on their properties are incomplete. The present study was designed to explore the interpolytype transitions induced by variation in the ambient humidity among LDHs. The cooperative behavior of intercalated water molecules resulted in a sudden, single-step, reversible dehydration of the [Zn-Cr-SO4] LDH. The [Zn-Al-SO4] LDH provided an interesting contrast with (1) the coexistence of the end members of the hydration cycle over the 40-20% relative humidity range during the dehydration cycle, and (2) a random interstratified intermediate in the hydration cycle. These observations showed that the [Zn-Al-SO4] LDH offered sites having a range of hydration enthalpies, whereby, at critical levels of hydration (20–40%), the non-uniform swelling of the structure resulted in an interstratified phase. The variation in domain size during reversible hydration was also responsible for the differences observed in the hydration vs. the dehydration pathways. This behavior was attributed to the distortion in the array of hydroxyl ions which departs from hexagonal symmetry on account of cation ordering as shown by structure refinement by the Rietveld method. This distortion was much less in the [Zn-Cr-SO4] LDH, whereby the nearly hexagonal array of hydroxyl ions offered sites of uniform hydration enthalpy for the intercalated water molecules. In this case, all the water molecules experienced the same force of attraction and dehydrated reversibly in a single step. The changes in basal spacing were also accompanied by interpolytype transitions, involving the rigid translations of the metal hydroxide layers relative to one another.
Similar content being viewed by others
References
Besserguenev, A.V., Fogg, A.M., Francis, R.J., Price, S.J., and O’Hare, D. (1997) Synthesis and structure of the gibbsite intercalated compounds [LiAl2(OH)2]X {X = Cl, Br, NO3}and [LiAl2(OH)6]Cl.H2O using synchronton X-ray and neutron powder diffraction. Chemistry of Materials, 9, 241–247.
Bigey, L., Depege, C., de Roy, A., and Besse, J.P. (1997) EXAFS and XANES study of layered double hydroxides. Journal de Physique IV, 7, 949–950.
Boclair, J.W., Braterman, P.S., Jiang, J., Lou, S., and Yarberry, F. (1999) Layered double hydroxides stability. 2. Formation of Cr (III)-containing layered double hydroxides directly from solution. Chemistry of Materials, 11, 303–307.
Boehm, H.P., Steinle, J., and Vieweger, C. (1977) [Zn2Cr(OH)6]x.2H2O, new layer compounds capable of anion exchange and intracrystalline swelling. Angewandte Chemie, 16, 265–266.
Breu, J., Seidl, W., Stoll, A.J., Lange, K.G., and Probst, T.U. (2001) Charge homogenity in synthetic fluorohectorite. Chemistry of Materials, 13, 4213–4220.
Cadars, S., Layrac, G., Gerardin, C., Deschamps, M., Yates, J.R., Tichit, D., and Massiot, D. (2011) Identification and quantification of defects in the cation ordering in Mg/Al layered double hydroxides. Chemistry of Materials, 23, 2821–2831.
Drits, V.A. and Bookin, A.S. (2001) Crystal structure and double hydroxides in water. Journal of Materials Chemistry, 15, 653–656.
Hofmeister, W. and Platen, H.V. (1992) Crystal chemistry and atomic order structures in brucite-related double-layer structure. Crystallography Reviews, 3, 3–26.
Hou, X. and Kirkpatrick, R.J. (2000) Solid-State 77Se NMR and XRD study of the structure and dynamic of seleno-oxyanions in hydrotalcite-like compounds. Chemistry of Materials, 12, 1890–1897.
Hou, X. and Kirkpatrick, R.J. (2002) Interlayer structure and dynamics of ClO -4 layered double hydroxides. Chemistry of Materials, 14, 1195–1200.
Hou, X., Bish, D.L., Wang, S.L., Johnston, C.T., and Kirkpatrick, R.J. (2003) Hydration, expansion, structure, and dyanamics of layered double hydroxides. American Mineralogist, 88, 167–179.
Iye, N., Fujii, K., Okamoto, K., and Sasaki, T. (2007) Factors influencing the hydration of layered double hydroxides (LDHs) and the appearance of an intermediate second staging phase. Applied Clay Science, 35, 218–227.
Khaldi, M., de Roy, A., Chaouch, M., and Besse, J.P. (1997) New varieties of zinc-chromium-sulfate lamellar double hydroxides. Journal of Solid State Chemistry. 130, 66–73.
Krivovichev, S.V., Yakovenchuk, V.N., Zhotova, E.S., Zolotarev, A.A., Pakhomovsky, Y.A., and Ivanyuk, G.Yu. (2010) Crystal chemistry of natural layered double hydroxides. I. Quintinite-2H-2c from Kovdor Alkaline Massif, Kola Peninsula, Russia. Mineralogical Magazine, 74, 821–832.
Larson, A.C. and Von Dreele, R.B. (2004) General Structure Analysis System (GSAS), Los Almos National Laboratory Report LAUR 86-748. Los Almos National Laboratory, Los Almos, NM.
Lasocha, W. and Lewiniski, K. (1994) PROSZKI, a system of programs for powder diffraction data analysis Version 2.4. Krakow, Poland.
Li, H., Ma, J., Evans, D.G., Zhou, T., Li, F., and Duan, X. (2006) Molecular dynamics modeling of the structure and binding energies of α-nickel hydroxides and nickel-aluminium layered double hydroxide containing various interlayer guest anions. Chemistry of Materials, 18, 4405–4414.
Mering, J. (1949) L’interfé rence des rayons X dans les systèmes à stratification désordonée. Acta Crystallographica, 2, 371–377.
Mostarih, R. and de Roy, A. (2006) Thermal behavior of a zinc-chromium-sulfate lamellar double hydroxide revisited as a function of vacuum and moisture parameter. Journal of Physics and Chemistry of Solids, 67, 1058–1062.
Oswald, H.R. and Asper, R. (1977) Preparation and Crystal Growth of Materials with Layered Structures (R.M.A. Leith, editor). Riedel Publishing Company, Dordrecht, The Netherlands, pp. 71–140.
Radha, S. and Kamath, P.V. (2013) Polytypism in sulfate-intercalated layered double hydroxides of Zn and M(III) (M = Al, Cr): Observation of cation ordering in the metal hydroxide layers. Inorganic Chemistry, 52, 4834–4841.
Radha, S., Milius, W., Breu, J., and Kamath, P.V. (2013) Synthesis and reversible hydration behavior of the thiosulfate intercalated layered double hydroxide of Zn and Al. Journal of Solid State Chemistry, 204, 362–366.
Reichle, W.T. (1986) Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics, 22, 135–141.
Roussel, H., Briois, V., Elkaim, E., de Roy, A., and Besse, J.P. (2000) Cationic order and structure of [Zn-Cr-Cl] and [Cu-Cr-Cl] layered double hydroxides: An XRD and EXAFS study. Journal of Physical Chemistry B, 104, 5915–5923.
Roussel, H., Briois, V., Elkaim, E., de Roy, A., Besse, J.P., and Jolivet, J.P. (2001) Study of the formation of the layered double hydroxide [Zn-Cr-Cl]. Chemistry of Materials, 13, 329–337.
Serna, C.J., Rendon, J.L., and Iglesias, J.E. (1982) Crystalchemical study of layered [Al2Li(OH)6]+X-·nH2O. Clays and Clay Minerals, 30, 180–184.
Sideris, P.J., Nielson, U.G., Gan, Z., and Grey, C.P. (2008) Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science, 321, 113–117.
Sideris, P.J., Blanc, F., Gan, Z., and Grey, C.P. (2012) Identification of cation clustering in Mg-Al layered double hydroxides using multinuclear solid state nuclear magnetic resonance spectroscopy. Chemistry of Materials, 24, 2449–2461.
Treacy, M.M.J., Newsam, J.M., and Deem, M.W. (1991) A general recursion method for calculating diffracted intensities from crystals containing planar faults. Proceedings of the Royal Society, London, A433, 499–520.
Treacy, M.M.J., Deem, M.W., and Newsam, J.M. (2000) Computer Code DIFFaX, Version 1.807. NEC Research Institute, Inc., Princeton, New Jersey, USA.
Vucelic, M., Jones, W., and Moggridge, G.D. (1997) Cation ordering in synthetic layered double hydroxides. Clays and Clay Minerals, 45, 803–813.
Weiss, A. (1963) A secret of Chinese porcelain manufacture. Angewandte Chemie, 75, 755–762.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Radha, S., Jayanthi, K., Breu, J. et al. Relative Humidity-Induced Reversible Hydration Of Sulfate-Intercalated Layered Double Hydroxides. Clays Clay Miner. 62, 53–61 (2014). https://doi.org/10.1346/CCMN.2014.0620105
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2014.0620105