Abstract
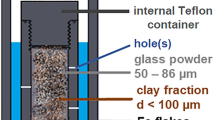
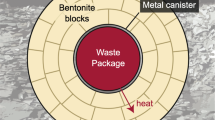
The design of the repository for high-level nuclear waste (HLW) in France consists of a multiple-barrier system including steel canisters in a clay host rock. The system will undergo temperature variations in time and space, the heat source being the HLW within the canisters. The effect of a thermal gradient in space on the Fe-claystone interaction was investigated here by applying a thermal gradient (150–300°C and 80–150°C) to a mix of claystone, Fe, and an aqueous chloride solution over periods of 3 and 6 months. Following the reaction, the starting clay minerals (mostly illite and mixed-layer illite-smectite) evolved toward chlorite, Fe-serpentine, Fe-saponite, mixed-layer chlorite-smectite, or mixed-layer serpentine-smectite as a function of temperature. Iron corrosion made the medium basic and reductive. Magnesium enrichment of clay minerals was observed in the hottest part of the experiment due to Mg migration under the thermal gradient. Reaction progress was enhanced at the lowest temperatures, compared to batch experiments.
Similar content being viewed by others
References
Bildstein, O., Trotignon, L., Perronnet, M., and Jullien, M. (2006) Modelling iron-clay interactions in deep geological disposal conditions. Physics and Chemistry of the Earth, Parts A/B/C, 31, 618–625.
Caillère, S., Hénin, S., and Rautureau, M. (1982) Minéralogie des Argiles. Structure et Propriétés Physico-Chimiques. Masson, Paris, 184 pp.
Calvert, C.C., Brown, A., and Brydson, R. (2005) Determination of the local chemistry of iron in inorganic and organic materials. Journal of Electron Spectroscopy and Related Phenomena, 143, 175–189.
Charpentier, D., Devineau, K., Mosser-Ruck, R., Cathelineau, M., and Villiéras, F. (2006) Bentonite-iron interactions under alkaline condition: An experimental approach. Applied Clay Science, 32, 1–13.
de Combarieu, G., Barboux, P., and Minet, Y. (2007) Iron corrosion in Callovo-Oxfordian argilite: From experiments to thermodynamic/kinetic modelling. Physics and Chemistry of the Earth, 32, 346–358.
de Combarieu, G., Schlegel, M.L., Neff, D., Foy, E., Vantelon, D., Barboux, P., and Gin, S. (2011) Glass-iron-clay interactions in a radioactive waste geological disposal: An integrated laboratory-scale experiment. Applied Geochemistry, 26, 65–79.
Gaucher, E., Robelin, C., Matray, J.M., Négrel, G., Gros, Y., Heitz, J.F., Vinsot, A., Rebours, H., Cassagnabère, A., and Bouchet, A. (2004) ANDRA underground research laboratory: interpretation of the mineralogical and geochemical data acquired in the Callovian-Oxfordian formation by investigative drilling. Physics and Chemistry of the Earth, Parts A/B/C, 29, 55–77.
Guillaume, D. (2002) Étude expérimentale du système fer — smectite en présence de solution á 80°C et 300°C. PhD thesis, Université Henri Poincaré, Nancy, France.
Guillaume, D., Neaman, A., Cathelineau, M., Mosser-Ruck, R., Peiffert, C., Abdelmoula, M., Dubessy, J., Villiéras, F., Baronnet, A., and Michau, N. (2003) Experimental synthesis of chlorite from smectite at 300°C in the presence of metallic Fe. Clay Minerals, 38, 281–302.
Guillaume, D., Neaman, A., Cathelineau, M., Mosser-Ruck, R., Peiffert, C., Abdelmoula, M., Dubessy, J., Villiéras, F., and Michau, N. (2004) Experimental study of the transformation of smectite at 80 and 300°C in the presence of Fe oxides. Clay Minerals, 39, 17–34.
Jodin-Caumon, M.-C., Mosser-Ruck, R., Rousset, D., Randi, A., Cathelineau, M., and Michau, N. (2010) Effect of a thermal gradient on iron-clay interactions. Clays and Clay Minerals, 58, 667–681.
Kostov, I. (1968) Mineralogy. Oliver and Boyd, Edinburgh and London, 587 pp.
Lantenois, S. (2003) Réactivité fer mé tal/smectites en milieu hydraté á 80°C. PhD thesis, Université d’Orléans, Orléans, France.
Lantenois, S., Lanson, B., Muller, F., Bauer, A., Jullien, M., and Plançon, A. (2005) Experimental study of smectite interaction with metal Fe at low temperature: 1. Smectite destabilization. Clays and Clay Minerals, 53, 597–612.
Madsen, F.T. (1998) Clay mineralogical investigations related to nuclear waste disposal. Clay Minerals, 33, 109–129.
Martin, F.A., Bataillon, C., and Schlegel, M.L. (2008) Corrosion of iron and low alloyed steel within a water saturated brick of clay under anaerobic deep geological disposal conditions: An integrated experiment. Journal of Nuclear Materials, 379, 80–90.
Marty, N.C.M., Fritz, B., Clément, A., and Michau, N. (2010) Modelling the long term alteration of the engineered bentonite barrier in an underground radioactive waste repository. Applied Clay Science, 47, 82–90.
Mosser-Ruck, R., Cathelineau, M., Guillaume, D., Charpentier, D., Rousset, D., Barres, O., and Michau, N. (2010) Effects of temperature, pH, and iron/clay and liquid/clay ratios on experimental conversion of dioctahedral smectite to berthierine, chlorite, vermiculite, or saponite. Clays and Clay Minerals, 58, 280–291.
Neaman, A., Guillaume, D., Pelletier, M., and Villiéras, F. (2003) The evolution of textural properties of Na/Cabentonite following hydrothermal treatment at 80 and 300°C in the presence of Fe and/or Fe oxides. Clay Minerals, 38, 213–223.
Olsson, S. and Karnland, O. (2011) Mineralogical and chemical characteristics of the bentonite in the A2 test parcel of the LOT field experiments at Äspö HRL, Sweden. Physics and Chemistry of the Earth, Parts A/B/C, 36, 1545–1553.
Osacký, M., Honty, M., Madejová, J., Bakas, T., and Šucha, V. (2009) Experimental interactions of Slovak bentonites with metallic iron. Geologica Carpathica, 60, 535–543.
Osacký, M., Šucha, V., Czímerová, A., and Madejová, J. (2010) Reaction of smectites with iron in a nitrogen atmosphere at 75°C. Applied Clay Science, 50, 237–244.
Perronnet, M., Jullien, M., Villiéras, F., Raynal, J., Bonnin, D., and Bruno, G. (2008) Evidence of a critical content in Fe(0) on F°Ca7 bentonite reactivity at 80°C. Applied Clay Science, 38, 187–202.
Pierron, O. (2011) Interactions eau-fer-argilite: Rôle des paramètres Liquide/Roche, Fer/Argilite, Température sur la nature des phases minérales. PhD Nancy Université. Université Henri Poincaré, Nancy, France.
Plötze, M., Kahr, G., Dohrmann, R., and Weber, H. (2007) Hydro-mechanical, geochemical and mineralogical characteristics of the bentonite buffer in a heater experiment: The HE-B project at the Mont Terri Rock Laboratory. Physics and Chemistry of the Earth, 32, 730–740.
Rivard, C. (2011) Contribution á l’’étude de la stabilité des minéraux constitutifs de l’fargilite du Callovo-Oxfordien en présence de fer á 90°C. PhD thesis, Nancy-Université — INPL, Nancy, France.
Savage, D., Watson, C., Benbow, S., and Wilson, J. (2010) Modelling iron-bentonite interactions. Applied Clay Science, 47, 91–98.
Schlegel, M.L., Bataillon, C., Benhamida, K., Blanc, C., Menut, D., and Lacour, J.-L. (2008) Metal corrosion and argillite transformation at the water-saturated, high-temperature iron-clay interface: A microscopic-scale study. Applied Geochemistry, 23, 2619–2633.
Schlegel, M.L., Bataillon, C., Blanc, C., Prêt, D., and Eddy, F. (2010) Anodic activation of iron corrosion in clay media under water-saturated conditions at 90°C: Characterization of the corrosion interface. Environmental Science & Technology, 44, 1503–1508.
Truche, L. (2009) Transformations minéralogiques et géochimiques induites par la présence d’hydrogène dans un site de stockage de déchets radioactifs. PhD thesis, Université Toulouse III Paul Sabatier, Toulouse, France.
Truche, L., Berger, G., Destrigneville, C., Guillaume, D., and Giffaut, E. (2010) Kinetics of pyrite to pyrrhotite reduction by hydrogen in calcite buffered solutions between 90 and 180°C: Implications for nuclear waste disposal. Geochimica et Cosmochimica Acta, 74, 2894–2914.
Vidal, O. (1997) Experimental study of the thermal stability of pyrophyllite, paragonite, and clays in a thermal gradient. European Journal of Mineralogy, 9, 123–140.
Vidal, O. and Durin, L. (1999) Aluminium mass transfer and diffusion in water at 400–550°C, 2 kbar in the K2O-Al2O3-SiO2-H2O system driven by a thermal gradient or by a variation of temperature with time. Mineralogical Magazine, 63, 633–647.
Vidal, O., Baldeyrou, A., Beaufort, D., Fritz, B., Geoffroy, N., and Lanson, B. (2012) Experimental study of the stability and phase relations of clays at high temperature in a thermal gradient. Clays and Clay Minerals, 60, 200–255.
Wilson, J., Cressey, G., Cressey, B., Cuadros, J., Ragnarsdottir, K.V., Savage, D. and Shibata, M. (2006a) The effect of iron on montmorillonite stability. (II) Experimental investigation. Geochimica et Cosmochimica Acta, 70, 323–336.
Wilson, J., Savage, D., Cuadros, J., Shibata, M. and Ragnarsdottir, K.V. (2006b) The effect of iron on montmorillonite stability. (I) Background and thermodynamic considerations. Geochimica et Cosmochimica Acta, 70, 306–322.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jodin-Caumon, MC., Mosser-Ruck, R., Randi, A. et al. Mineralogical Evolution of a Claystone After Reaction With Iron Under Thermal Gradient. Clays Clay Miner. 60, 443–455 (2012). https://doi.org/10.1346/CCMN.2012.0600501
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2012.0600501