Abstract
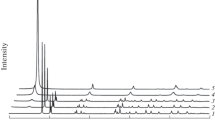
The role of Mn oxide in the abiotic formation of humic substances has been well demonstrated. However, information on the effect of crystal structure and surface-chemical characteristics of Mn oxide on this process is limited. In the present study, hexagonal and triclinic birnessites, synthesized in acidic and alkali media, were used to study the influence of the crystal-structure properties of birnessites on the oxidative polymerization of hydroquinone and to elucidate the catalytic mechanism of birnessites in the abiotic formation of humic-like polymers in hydroquinone-birnessite systems. The intermediate and final products formed in solution and solid-residue phases were identified by UV/Visible spectroscopy, atomic absorption spectrometry, Fourier-transform infrared spectroscopy, X-ray diffraction, solid-phase microextraction-gaschromatography-mas ss pectrometry, ion chromatography, and ultrafiltration. The degree of oxidative polymerization of hydroquinone wasenhanced with increase in the interlayer hydrated H+, the average oxidation state (AOS), and the specific surface area of birnessites. The nature of the functional groups of the humic-like polymers formed was, however, almost identical when hydroquinone was catalyzed by hexagonal and triclinic birnessites with similar AOS of Mn. The results indicated that crystal structure and surface-chemistry characteristics have significant influence on the oxidative activity of birnessites and the degree of polymerization of hydroquinone, but have little effect on the abiotic formation mechanism of humic-like polymers. The proposed oxidative polymerization pathway for hydroquinone isthat, asit approachesthe birnessite, it formsp recursor surface complexes. Asa strong oxidant, birnessite accepts an electron from hydroquinone, which is oxidized to 1,4-benzoquinone. The coupling, cleavage, polymerization, and decarboxylation reactionsaccompany the generation of 1,4-benzoquinone, lead to the release of CO2 and carboxylic acid fragments, the generation of rhodochrosite, and the ultimate formation of humic-like polymers. These findings are of fundamental significance in understanding the catalytic role of birnessite and the mechanism for the abiotic formation of humic substances in nature.
Similar content being viewed by others
References
Ahn, M.-Y., Martinez, C.E., Archibald, D.D., Zimmerman, A.R., Bollag, J.-M., and Dec, J. (2006) Transformation of catechol in the presence of a laccase and birnessite. Soil Biology & Biochemistry, 38, 1015–1020.
Aktas, N., Sahiner, N., Kantoglu, O., Salih, B., and Tanyolac, A. (2003) Biosynthesis and characterization of laccase catalyzed poly(catechol). Journal of Polymers and ther Environment, 11, 123–128.
Bartlett, R.J. and Ross, D.S. (2005) Chemistry of redox processes in soils. Pp. 461–487 in: Chemical Processes in Soils (M.A. Tabatabai, D.L. Sparks, L. Al-Amoodi, and W.A. Dick, editors). Soil Science Society of America, Madison, Wisconsin, USA.
Bosetto, M., Arfaioli, P., and Pantani, O.L. (2002) Study of the Maillard reaction productsf ormed by glycine and D-glucose on different mineral substrates. Clay Minerals, 37, 195–204.
Brunauer, S., Emmett, P.H., and Teller, E. (1938) Adsorption of gases in multi molecular layers. Journal of the American Chemical Society, 60, 309–319.
Colarieti, M.L., Toscano, G., Ardi, M.R., and Greco, Jr. G. (2006) Abiotic oxidation of catechol by soil metal oxides. Journal of Hazardous Materials, 134, 161–168.
Dec, J., Haider, K., and Bollag, J. (2001) Decarboxylation and demethoxylation of naturally occurring phenolsdu ring coupling reactionsand polymerization. Soil Science, 166, 660–671.
Drits, V.A., Silvester, E., Gorshkov, A.I., and Manceau, A. (1997) Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite: I. Results from X-ray diffraction and selected-area electron diffraction. Amercian Mineralogist, 82, 946–961.
Gonzalen, J.M. and Laird, D.A. (2004) Role of smectites and Al-substituted goethites in the catalytic condensation of arginine and glucose. Clays and Clay Minerals, 52, 443–450.
Hardie, A.G. (2008) Pathwaysof abiotic humification as catalyzed by mineral colloids. PhD thesis, University of Saskatchewan, Saskatoon, Canada, 255 pp.
Hardie, A.G., Dynes, J.J., Kozak, L.M., and Huang, P.M. (2007) Influence of polyphenolson the intergrated polyphenol-Maillard reaction humification pathway ascatalyzed by birnessite. Annals of Environmental Science, 1, 91–110
Hardie, A.G., Dynes, J.J., Kozak, L.M., and Huang, P.M. (2009a) The role of glucose in abiotic humification pathwaysascatalyzed by birnessite. Journal of Molecular Catalysis A — Chemical, 308, 114–126.
Hardie, A.G., Dynes, J.J., Kozak, L.M., and Huang, P.M. (2009b) Biomolecule-induced carbonate genesis in abiotic formation of humic substances in nature. Canadian Journal of Soil Science, 89, 445–453.
Huang, P.M. and Hardie, A.G. (2009) Formation mechanisms of humic substances in the environment. Pp. 41–109 in: Biophysico-chemical Processes Involving Nonliving Natural Organic Matter in the Environment. Vol. 2 (N. Senesi, B. Xing and P.M. Huang, editors). Wiley-IUPAC series Biophysico-Chemical Processes in Environmental Systems. John Wiley & Sons, Hoboken, New Jersey, USA.
Jokic, A. (2000) The catalytic role of birnessite in the Maillard reaction and the abiotic formation of humic substances. PhD thesis, University of Saskatchewan, Saskatoon, Canada, 184 pp.
Jokic, A., Frenkel, A.I., and Huang, P.M. (2001a) Effect of light on birnessite catalysis of the Maillard reaction and its implication in humification. Canadian Journal of Soil Science, 81, 277–283
Jokic, A., Frenkel, A.I., Vairavamurthy, M.A., and Huang, P.M. (2001b) Birnessite catalysis of the Maillard reaction: itss ignificance in natural humification. Geophysical Research Letters, 28, 3899–3902.
Jokic, A., Wang, M.C., Liu, C., Frenkel, A.I., and Huang, P.M. (2004) Integration of the polyphenol and Maillard reactions into a unified abiotic pathway for humification in nature: the role of δ-MnO2. Organic Geochemistry, 35, 747–762.
Kappler, A., Ji, R., and Brune, A. (2000) Synthesis and characterization of specifically 14C-labeled humic model compoundsfor feeding trials with soil-feeding termites. Soil Biology & Biochemistry, 32, 1271–1280.
Kijima, N., Yasuda, H., Sato, T., and Yoshimura, Y. (2001) Preparation and characterization of open tunnel oxide α-MnO2 precipitated by ozone oxidation. Journal of Solid State Chemistry, 159, 94–102.
Kilduff, J.E. and Weber, W.J. (1992) Transport and separation of organic macromolecules in ultrafiltration processes. Environmental Science & Technology, 26, 569–577.
Kim, J.B., Dixon, J.B., and Chusuei, C.C. (2002) Oxidation of chromium(III) to (VI) by manganese oxides. Soil Science Society of America Journal, 66, 306–315.
Lanson, B., Drits, V.A., Silvester, E., and Manceau, A. (2000) Structure of H-exchanged hexagonal birnessite and its mechanism of formation from Na-rich monoclinic buserite at low pH. American Mineralogist, 85, 826–838.
Lanson, B., Drits, V.A., Feng, Q., and Manceau, A. (2002) Structure of synthetic Na-birnessite: Evidence for a triclinic one-layer unit cell. American Mineralogist, 87, 1662–1671.
Lee, J.S.K. and Huang, P.M. (1995) Photochemical effect on the abiotic transformationsof polyphenolicsas catalyzed by Mn(IV) oxide. Pp. 177–189 in: Environmental Impact of Soil Component Interactions. Vol 1. Natural and Anthropogenic Organics (P.M. Huang, J. Berthelin, J.-M. Bollag, W.B. McGill, and A.L. Page, editors). Lewis Publishers, Boca Raton, Florida, USA.
Li, L., Zhao, Z.Y., Huang, W.L., Peng, P., Sheng, G.Y., and Fu, J.M. (2004) Characterization of humic acidsf ractionated by ultrafiltration. Organic Geochemistry, 35, 1025–1037.
Liu, C. and Huang, P.M. (2001) The influence of catechol humification on surface properties of metal oxides. Pp. 253–270 in: Humic Substances: Structure, Models and Functions (E.A. Ghabbour and G. Davies, editors). Royal Society of Chemistry, Cambridge, UK.
Liu, C. and Huang, P.M. (2002) Role of hydroxy-aluminosilicate ions(proto-imogolite sol) in the formation of humic substances. Organic Geochemistry, 33, 295–305.
Liu, M.M., Zeng, Z.R., and Fang, H.F. (2005) Preparation and application of the sol-gel-derived acrylate/silicone copolymer coatings for headspace solid-phase microextraction of 2-chloroethyl ethyl sulfide in soil. Journal of Chromatography A, 1076, 16–26.
Majcher, E.H., Chorover, J., Bollag, J.M., and Huang, P.M. (2000) Evolution of CO2 during birnessite-induced oxidation of C-14-labeled catechol. Soil Science Society of America Journal, 64, 157–163.
Matocha, C.J., Sparks, D.L., Amonette, J.E., and Kukkadapu, R.K. (2001) Kinetics and mechanism of birnessite reduction by catechol. Soil Science Society of America Journal, 65, 58–66.
McBride, M.B. (1994) Environmental Chemistry of Soils. Oxford University Press, New York.
McKenzie, R.M. (1971) The synthesis of birnessite, cryptomelane, and some other oxidesand hydroxidesof manganese. Mineralogical Magazine, 38, 493–502.
Nico, P.S. and Zasoski, R.J. (2000) Importance of Mn(III) availability on the rate of Cr(III) oxidation on δ-MnO2. Environmental Science & Technology, 34, 3363–3367.
Nico, P.S. and Zasoki, R.J. (2001) Mn(III) center availability asa rate controlling factor in the oxidation of phenol and sulfide on δ-MnO2. Environmental Science & Technology, 35, 3384–3343.
Potter, R.M. and Rossman, G.R. (1979) The tetravalent manganese oxides: identification, hydration, and structural relationships by infrared spectroscopy. American Mineralogist, 64, 1199–1218.
Scheffer, F., Meyer, B., and Niederbudde, E. (1959) Huminstoflbildung unter katalyischer Einwirkung naturlich vorkornmender Eisenverbindungen im ModelIversuch. Zeitschrift für Pflanzenernährung und Bodenkunde, 87, 26–44.
Shindo, H. and Huang, P.M. (1982) Role of manganese(IV) oxide in abiotic formation of humic substances in the environment. Nature, 298, 363–365.
Shindo, H. and Huang, P.M. (1985) The catalytic power of inorganic components in the abiotic synthesis of hydroquinone-derived humic polymers. Applied Clay Science, 1, 71–81.
Shindo, H. and Huang, P.M. (1992) Comparison of the influence of Mn(IV) oxide and tyrosinase on the formation of humic substances in the environment. Science of the Total Environment, 117/118, 103–110.
Stone, A.T. (1987) Reductive dissolution of manganese (III/IV) oxides by substituted phenols. Environmental Science & Technology, 21, 979–986.
Tan, J.F., Qiu, G.H., Liu, F., Tan, W.F., and Feng, X.H. (2009) Effectsof Mn (III) on oxidation of Cr (III) with birnessite. Huanjing Kexue/Environmental Science (China), 30, 2779–2785.
Tan, W., Koopal, L., Weng, L., Vanriemsdijk, W., and Norde, W. (2008) Humic acid protein complexation. Geochimica et Cosmochimica Acta, 72, 2090–2099.
Villalobos, M., Toner, B., Bargar, J., and Sposito, G. (2003) Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochimica et Cosmochimica Acta, 67, 2649–2662.
Villalobos, M., Lanson, B., Manceau, A., Toner, B., and Sposito, G. (2006) Structural model for the biogenic Mn oxide produced by Pseudomonas putida. American Mineralogist, 91, 489–502.
Wang, M.C. and Huang, P.M. (1992) Significance of Mn(IV) oxide in the abiotic ring cleavage of pyrogallol in natural environments. Science of the Total Environment, 113, 147–157.
Wang, M.C. and Huang, P.M. (2000) Ring cleavage and oxidative transformation of pyrogallol catalyzed by Mn, Fe, Al, and Si oxides. Soil Science, 165, 934–942.
Wang, M.C. and Huang, P.M. (2003) Cleavage and polycondensation of pyrogallol and glycine catalyzed by natural soil clays. Geoderma, 112, 31–50.
Wang, M.C. and Huang, P.M. (2005) Cleavage of 14C-labeled glycine and itspoly condensation with pyrogallol as catalyzed by birnessite. Geoderma, 124, 415–426.
Wilson, W.R. (1991) Advances in Soil Organic Matter Research: The Impact on Agricul ture and the Environment. Royal Society of Chemistry, Cambridge, UK.
Zavarzina, A.G. (2006) A mineral support and biotic catalyst are essential in the formation of highly polymeric soil humic substances. Eurasian Soil Science, 39 (Suppl. 1), S48–S53.
Zhao, W., Feng, X., Tan, W., Liu, F., and Ding, S. (2009a) Relation of lead adsorption on birnessites with different average oxidation states of manganese and release of Mn2+/H+/K+. Journal of Environmental Sciences, 21, 520–526.
Zhao, W., Cui, H.J., Liu, F., Tan, W.F., and Feng, X.H. (2009b) Relation between Pb2+ adsorption and average Mn oxidation state in synthetic birnessites. Clays and Clay Minerals, 57, 513–520.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, MM., Cao, XH., Tan, WF. et al. Structural Controls on the Catalytic Polymerization of Hydroquinone by Birnessites. Clays Clay Miner. 59, 525–537 (2011). https://doi.org/10.1346/CCMN.2011.0590510
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2011.0590510