Abstract
In spite of many studies of kaolinite synthesis, questions remain as to the transformation of gel into kaolinite, the kinetics of the reaction, and the influence of solution chemistry. The purpose of the present study was to perform a hydrothermal synthesis in order to understand better the transformation from boehmite to kaolinite. Kaolinite was synthesized from amorphous SiO2 and Al(OH)3·xH2O at fixed temperature (250°C) and pressure (30 bar). The initial pH of the solution was 2. The reaction time for the synthesis was varied from 2 to 36 h. The physical properties of synthesized kaolinite were characterized by X-ray diffraction (XRD), infrared spectroscopy (IR), nuclear magnetic resonance (NMR) spectroscopy, field-emission-scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), and energy dispersive spectrometry (EDS).
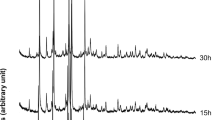
The early stage of kaolinite synthesis followed activation of amorphous Al(OH)3·xH2O to initiate the reactions, i.e. ionization and subsequent crystallization of boehmite. The boehmite reacted continuously with Si4+ dissolved in solution and gradually transformed to disordered, lath-shaped boehmite. In XRD and IR patterns, the typical peaks of boehmite were weakened or disappeared following the reaction.
Structural transformation from boehmite to kaolinite occurred when the Al/Si ratio of the aluminosilicate was 1.0. The kaolinite formed was in the form of curved flakes and its crystallinity increased with reaction time. In the final stage of reaction the morphology of kaolinite changed from flaky to polygonal. The hexagonal, platy kaolinite was therefore developed to allow the gradual variation of the chemical composition, crystal structure, and morphology.
Similar content being viewed by others
References
Brindly, G.W., Santos, P.S., and Santos, H.S. (1963) Mineralogical studies of kaolinite-halloysite clays: Part I, Identification problems. American Mineralogist, 48, 897–910.
Eberl, D.D. and Hower, J. (1975) Kaolinite synthesis; the role of the SiAl and (alkali)/(H+) ratio in hydrothermal systems. Clays and Clay Minerals, 23, 301–309.
Farmer, V.C. (1974) The Infrared Spectra of Minerals. Monograph 4, Mineralogical Society, London.
Fialips, C.I., Petit, S., Decarreau, A., and Beaufort, D. (2000) Influence of synthesis pH on kaolinite crystallinity and surface properties. Clays and Clay Minerals, 48, 173–184.
Fiore, S., Huertas, F.J., Huertas, F., and Linares, J. (1995) Morphology of kaolinite crystals synthesized under hydrothermal conditions. Clays and Clay Minerals, 43, 353–360.
Huertas, F.J., Huertas, F., and Linares, J. (1993) Hydrothermal synthesis of kaolinite: method and characterization of synthetic materials. Applied Clay Science, 7, 345–356.
Huertas, F.J., Fiore, S., Huertas, F., and Linares, J. (1999) Experimental study of the hydrothermal formation of kaolinite. Chemical Geology, 156, 171–190.
Huertas, F.J., Fiore, S., and Linares, J. (2004) In-situ transformation of amorphose gel into spherical aggregates of kaolinite: a TEM study. Clay Minerals, 39, 423–431.
Kirkpatrick, R.J. and Phillips, B.L. (1993) 27Al NMR spectroscopy of minerals and related materials. Applied Magnetic Resonance, 4, 213–236.
Kittrick, J.A. (1966) The free energy of formation of gibbsite and Al(OH) −4 from solubility measurements. Soil Science Society of America Proceedings, 30, 595–598.
La Iglesia, A. and Martin Vivaldi, J.L. (1972) A contribution to the synthesis of kaolinite. In: Proceedings of the International Clay Conference, Madrid, pp. 173–185.
La Iglesisa, A. and Martin-Vivaldi, J.L. (1975) Synthesis of kaolinite by homogeneous precipitation at room temperature: I. Use of anionic resin in (OH) form. Clay Minerals. 10, 399–405.
La Iglesia, A., Martin-Vivaldi, J.L. and Lopez-Aguayo, F., Jr. (1976) Kaolinite crystallization at room temperature by homogeneous precipitation: III. Hydrolysis of feldspars. Clays and Clay Minerals, 24, 36–42.
Mason, B. (1966) Principles of Geochemistry, 3rd edition. John Wiley and Sons, Inc., New York, pp. 164–167.
Nagy, K.L. (1995) Dissolution and precipitation kinetics of sheet silicates. Pp. 173–233 in: Chemical Weathering Rates of Silicate Minerals (A.F. White and S.L. Brantley, editors). Reviews in Mineralogy, 31. Mineralogical Society of America, Washington, D.C.
Rayner, J.H. (1962) An examination of the rate of formation of kaolinite from a coprecipitated silica gel. In: Colloque sur la Gènese et al Synthèse des Argilles, C.N.R.S., No. 105. Paris, pp. 123–127.
Robins, R.G. (1967) Hydrothermal precipitation in solutions of thorium nitrate, ferric nitrate and aluminium nitrate. Journal of Inorganic Nuclear Chemistry, 29, 431–435.
Rusell, J.D. (1987) Infrared methods. Pp. 133–172 in: A Handbook of Determinative Methods in Clay Mineralogy (M.J. Wilson, editor). Chapman & Hall, London.
Ryu, K.W., Jang, Y.N., Chae, S.C., Bae, I.K., and Lee, S.K. (2008) The characterization of kaolinite synthesized according to the pH. Korea Society of Economic and Environmental Geology, 41, 165–172.
Satokawa, S., Osaki, Y., Samejima, S., Miyawaki, R., Tomura, S., Shibasaki, Y., and Sugahara, Y. (1994) Effects of the structure of silica-alumina gel on the hydrothermal synthesis of kaolinite. Clays and Clay Minerals, 42, 288–297.
Satokawa, S., Miyawaki, R., Osaki, Y., Tomura, S., and Shibasaki, Y. (1996) Effects of acidity on the hydrothermal synthesis of kaolinite from silica-gel and gibbsite. Clays and Clay Minerals, 44, 417–423.
Slade, R.C.T. and Davies, T.W. (1989) The mechanism of kaolinite dehydroxylation followed by high resolution 27Al NMR and 29Si NMR. Colloids and Surfaces, 36, 119–125.
Small, J.S. (1993) Experimental determination of the rates of precipitation of authigenic illite and kaolinite in the presence of aqueous oxalate and comparison to the K/Ar ages of authigenic illite in reservoir sandstones. Clays and Clay Minerals, 41, 191–208.
Small, J.S., Hamilton, D.L., and Habesch, S. (1992). Experimental simulation of clay precipitation within reservoir sandstones: 1. Techniques and examples. Journal Of Sedimentary Petrology, 62, 508–519.
Stiffert, B. and Wey, R. (1972) Contribution a la connaissance de lasynthese des kaolins. In: Proceeding of International Clay Conference, Madrid, pp. 159–172.
Tomura, S., Shibasaki, Y., Mizuta, H., and Kitamura, M. (1985) Growth conditions and genesis of spherical and platy kaolinite. Clays and Clay Minerals, 33, 200–206.
Van der Marel, H.W. and Beutelspacher, H. (1976) Atlas of Infrared Spectroscopy of Clay Minerals and their Admixtures. Elsevier, Amsterdam.
Zhang, X.R. and Xu, Z. (2007) The effect of microwave on preparation of kaolinite/dimethylsulfoxide composite during intercalation process. Materials Letters, 61, 1478–1482.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, KW., Jang, YN. & Chae, SC. Hydrothermal Synthesis of Kaolinite and its Formation Mechanism. Clays Clay Miner. 58, 44–51 (2010). https://doi.org/10.1346/CCMN.2010.0580104
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2010.0580104