Abstract
Natural and synthetic micas have been used widely as substrates to study biological systems; but, as in the case of negatively charged DNA, anionic charge repulsion may render micas a less than ideal templating surface for many biological systems. The purpose of this study was to investigate the potential for the chlorite clinoclore, which contains a positively charged interlayer octahedral sheet, to serve as a substrate for DNA adsorption. The relationships between clinochlore cleavage characteristics, in terms of nano-morphology, and surface potential are investigated, as are its average crystal chemistry and topology. That the structural features of clinochlore can be used successfully to condense, order, and self assemble complex biomolecules, such as DNA is also proven.
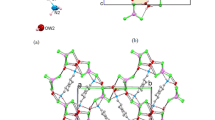
A natural IIb-4 clinochlore [\(C\bar 1\) symmetry, unit-cell parameters a = 0.53301(4); b = 0.92511(6); c = 1.4348(1) (nm); α = 90.420(3); β = 97.509(3); γ = 89.996(4) (°)] with chemical composition \(\left( {{\rm{M}}{{\rm{g}}_{1.701}}{\rm{Fe}}_{0.245}^{2 + }{\rm{T}}{{\rm{i}}_{0.004}}{\rm{A}}{{\rm{l}}_{0.998}}{\rm{Cr}}_{0.052}^{3 + }} \right)\;{\rm{M}}{{\rm{g}}_3}\;\left( {{\rm{S}}{{\rm{i}}_{2.939}}\;{\rm{A}}{{\rm{l}}_{1.015}}\;{\rm{Fe}}_{0.046}^{3 + }} \right){{\rm{O}}_{10}}\left( {{\rm{O}}{{\rm{H}}_{7.913}}{{\rm{F}}_{0.087}}} \right)\) was selected. The octahedral sites of the silicate layer (<M(1)−O> = 0.2080 nm and <M(2)−O> = 0.2081 nm) are equal and occupied by Mg, whereas the octahedral sites in the interlayer M(3) and M(4) (<M(3)−O> = 0.2088 nm and <M(4) − O> = 0.1939 nm) show different sizes and are mostly completely occupied by divalent (Mg2+ and Fe2+) and trivalent (Al3+) cations, respectively.
The clinochlore cleaved surface is present in two forms: (1) the stripe type (0.40 nm in height, up to several micrometers long and ranging from some nanometers to a few microns in lateral size); and (2) the triangular type (0.40 nm in height). Both features may result either from interlayer sheets whose cleavage weak directions are related to the different M(3) and M(4) site occupancy, or from weak interlayer bonding along specific directions to the 2:1 layer underneath. The cleaved surface, particularly at the cleaved edges, presents high DNA affinity, which is directly related to an average positive surface and ledge potential.
Similar content being viewed by others
References
Antognozzi, M., Szczelkun, M., Round, A.N., and Miles, M.J. (2002) Comparison between shear force and tapping mode AFM — high resolution imaging of DNA. Single Molecules, 3, 105–110.
Antognozzi, M., Wotherspoon, A., Hayes, J.M., Miles, M.J., Szczelkun, M.J., and Valdrè, G. (2006) A chlorite mineral surface actively drives the deposition of DNA molecules in stretched conformations. Nanotechnology, 17, 3897–3902.
Bailey, S.W. (1988) Chlorites: structures and crystal chemistry. Pp. 347–403 in: Hydrous Phyllosilicates (exclusive of micas) (S.W. Bailey, editor). Reviews in Mineralogy, 19, Mineralogical Society of America, Chantilly, Virginia.
Bayliss, P. (1975) Nomenclature of the trioctahedral chlorites. The Canadian Mineralogist, 13, 178–180.
Bruker (2003a) APEX2. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (2003b) SAINT-IRIX. Bruker AXS Inc., Madison, Wisconsin, USA.
Bustamante, C., Vesenka, J., Tang, C.L., Rees, W., Guthold, M., and Keller, R. (1992) Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry, 31, 22–26.
Chang, D.-K. and Cheng, S.-F. (1996) On the importance of van der Waals interaction in the groove binding of DNA with ligands: restrained molecular dynamics study. International Journal of Biological Macromolecules, 19, 279–285.
Downs, R.T. and Hazen, R.M. (2004) Chiral indices of crystalline surfaces as a measure of enantioselective potential. Journal of Molecular Catalysis A: Chemical, 216, 273–285.
Giuli, G., Paris, E., Wu, Z.Y., Brigatti, M.F., Cibin, G., Mottana, A., and Marcelli, A. (2001) Experimental and theoretical XANES and EXAFS study of tetra-ferriphlogopite. European Journal of Mineralogy, 13, 1099–1108.
Ha, B.Y. and Liu, A.J. (1997) Counterion-mediated attraction between two like-charged rods. Physical Review Letters, 79, 1289–1292.
Joshi, M.S. and Paul, B.K. (1977) Surface structures of trigonal bipyramidal faces of natural quartz crystals. American Mineralogist, 62, 122–126.
Joshi, M.S., Kotru, P.N., and Ittiakhen, M.A. (1970) Studying dislocations in quartz by the hydrothermal-etching method. Soviet Physics Crystallography, 15, 83–89.
Joswig, W. and Fuess, H. (1990) Refinement of a one-layer triclinic chlorite. Clays and Clay Minerals, 38, 216–218.
Klinov, D., Dwir, B., Kapon, E., Borovok, N., Molotsky, T., and Kotlyar, A. (2006) Comparative study of atomic force imaging of DNA on graphite and mica surfaces. American Institute of Physics Conference Proceedings, 859, 99–106.
Krause, M.O. and Oliver, J.H. (1979) Natural widths of atomic K and L Levels, Kα X-ray lines and several KLL Auger lines. Journal of Physical and Chemical Reference Data, 8, 329–338.
Lougear, A., Grodzicki, M., Bertoldi, C., Trautwein, A.X., Steiner, K., and Amthauer, G. (2000) Mössbauer and molecular orbital study of chlorites. Physics and Chemistry of Minerals, 27, 258–269.
Meyrowitz, R. (1970) New semi-microprocedure for determination of ferrous iron in refractory silicate minerals using a sodium metafluoroborate decomposition. Analytical Chemistry, 42, 1110–1113.
Parsons, R. (1990) Electrical double layer: Recent experimental and theoretical developments. Chemical Reviews, 90, 813–826.
Sheldrick, G.M. (2005) SADABS. Version 2.10. University of Göttingen, Germany.
Sheldrick, G.M. (1997) SHELX-97, program for crystal structure determination. University of Göttingen, Germany.
Sugimura, H., Ishida, Y., Hayashi, K., Takai, O., and Nakagiri, N. (2002) Potential shielding by the surface water layer in Kelvin probe force microscopy. Applied Physics Letters, 80, 1459–1461.
Sushko, M.L., Shluger, A.L., and Rivetti, C. (2006) Simple model for DNA adsorption onto a mica surface in 1:1 and 2:1 electrolyte solution. Langmuir, 22, 7678–7688.
Theng, H.H., Dove, P.M., Orme, C.A., and DeYoreo, J.J. (1998) The thermodynamics of calcite growth: A baseline for understanding biomineral formation. Science, 282, 724–727.
Tombolini, F., Brigatti, M.F., Marcelli, A., Cibin, G., Mottana, A., and Giuli, G. (2002) Crystal chemical study by XANES of trioctahedral micas: the most characteristic layer silicates. International Journal of Modern Physics B: Condensed Matter Physics, Statistical Physics, Applied Physics, 16, 1673–1679.
Valdrè, G. (2005) AFM observation of agglomerates, ordered structures and filaments after deposition of DNA nucleotides onto layer silicate mineral structures. Scanning, 27, 100–102.
Valdrè, G. (2007) Natural nanoscale surface potential of clinochlore and its ability to align nucleotides and drive DNA conformational change. European Journal of Mineralogy, 19, 309–319.
Valdrè, G., Antognozzi, M., Wotherspoon, A., and Miles, M.J. (2004) Influence of properties of layered silicate minerals on adsorbed DNA surface affinity, self-assembly and nanopatterning. Philosophical Magazine Letters, 84, 539–545.
Vesenka, J., Guthold, M., Tang, C.L., Keller, D., Delaine, E., and Bustamante, C. (1992) A substrate preparation for reliable imaging of DNA molecules with the scanning force microscope. Ultramicro s copy, 42–44, 1243–1249.
Waychunas, G.A. (1987) Synchrotron radiation XANES spectroscopy of titanium in minerals: Effects of titanium bonding distances, titanium valence, and site geometry on absorption edge structure. American Mineralogist, 72, 89–101.
Wilson, A.J.C. and Prince, E., editors (1999) International Tables for X-ray Crystallography, Volume C: Mathematical, physical and chemical tables (2nd edition). Kluwer Academic, Dordrecht, The Netherlands.
Wu, Z., Mottana, A., Marcelli, A., Natoli, C.R., and Paris, E. (1996) Theoretical analysis of X-ray absorption near-edge structure in forsterite, Mg2SiO4-Pbnm, and fayalite, Fe2SiO4-Pbnm, at room temperature and extreme conditions. Physics and Chemistry of Minerals, 23, 193–204.
Wu, Z., Natoli, C.R., Marcelli, A., Paris, E., Seifert, F., Zhang, J., and Liu, T. (2001) Symmetry role on the pre-edge X-ray absorption fine structure at the metal K edge. Journal of Synchrotron Radiation, 8, 215–217.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valdrè, G., Malferrari, D. & Brigatti, M.F. Crystallographic features and cleavage nanomorphology of chlinochlore: Specific applications. Clays Clay Miner. 57, 183–193 (2009). https://doi.org/10.1346/CCMN.2009.0570205
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2009.0570205