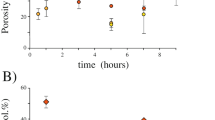
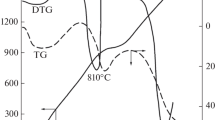
Abstract
The formation of hard hematite in steam generators with relatively high levels (5–10 µg/L) of dissolved oxygen at temperatures around 280–290°C and pressures around 6–8 MPa can serve as an analog for the formation of hard hematite in sedimentary processes. Furthermore, in steam generators, as well as in nature, hematite is an effective cementing agent, capable of incorporating as much as twice its own weight of other solids to form a hard composite material. Laboratory simulations showed ferrihydrite to be the likely starting material for the formation of hard, dense hematite at temperatures much lower than those required for sintering of anhydrous hematite. These laboratory simulations, performed at temperatures around 260°C and pressures of ∼500 MPa, resulted in the formation of hard hematite or hematite-based composite solids over periods of 3–5 h, compared with several months in steam generators and many years in nature. The amount of water present during the synthesis (10–15% of the weight of dry ferrihydrite) and the gradual removal of water proved to be key parameters in the formation of hard, dense hematite. The mechanism, studied by means of X-ray diffractometry, Mössbauer spectroscopy and infrared spectroscopy, appeared to involve build-up, then gradual condensation of OH bridges, leading to the conversion of ferrihydrite to hydrohematite with approximately 4–5% of residual water. The presence of other solids, such as copper and its oxides, alumina and silica, in large quantities, resulted in smaller grain size of the hydrohematite product but did not affect its mechanical properties. On the other hand, the use of hydrazine to provide a reducing environment produced goethite during the precursor synthesis stage and soft magnetite during the pressing stage. However, whenever hematite was produced, it could not be subsequently reduced to magnetite by hydrazine under the reaction conditions specified above. The mechanical properties as well as the spectroscopic characteristics of the product of pressing agreed with observations on sedimentary hematite-cemented rocks.
Similar content being viewed by others
References
Bagchi, T.P. and Sen, P.K. (1982) Kinetics of densification of powder compacts during the initial stage of sintering with constant rates of heating. A thermal analysis approach. Thermochimica Acta, 56, 269–283.
Barron, V., Rendon, J.L., Torrent, J. and Serna, C.J. (1984) Relation of infrared, crystallochemical, and morphological properties of Al-substituted hematites. Clays and Clay Minerals, 32, 475–479.
Burns, R., Cherepakhov, G., Mark, J.T. and Skulte, P. (1996) Indian Point 2 Station. Pp. A-43–A-47 in: PWR Secondary Water Chemistry Guidelines — Revision 4, EPRI TR-102134-R4, (A. McIlree, P. Millett and C.J. Wood, editors). Electric Power Research Institute, Palo Alto, California, USA.
Campbell, A.S., Schwertmann, U. and Campbell, P.A. (1997) Formation of cubic phases on heating ferrihydrite. Clay Minerals, 32, 615–622.
Carlson, L. and Schwertman, U. (1981) Natural ferrihydrites in surface deposits from Finland and their association with silica. Geochimica et Cosmochimica Acta, 45, 421–429.
Childs, C.W. (1992) Ferrihydrite: A review of structure, properties and occurrence in relation to soils. Zeitschrift fuer Pflanzenernaehrung und Bodenkunde, 155, 441–448.
Cornejo, J. (1986) The effect of heat treatment on textural evolution of ferrihydrite. Materials Chemistry and Physics, 15, 369–377.
Cornell, R.M. and Giovanoli, R. (1988) The influence of copper on the transformation of ferrihydrite (5Fe2O3·9H2O) into crystalline products in alkaline media. Polyhedron, 7, 385–391.
Cornell, R.M. and Schwertmann, U. (2003) The Iron Oxides, 2nd edition. Wiley-VCH, Weinheim, Germany.
Cornell, R.M., Giovanoli, R. and Schindler, P.W. (1987) Effect of silicate species on the transformation of ferrihydrite into goethite and hematite in alkaline media. Clays and Clay Minerals, 35, 21–28.
Cornell, R.M., Giovanoli, R. and Schneider, W. (1989) Review of the hydrolysis of iron(III) and the crystallization of amorphous iron(III) hydroxide hydrate. Journal of Chemical Technology and Biotechnology, 46, 115–134.
Crosby, W.O. (1891) On the contrast in color of the soils of high and low latitudes. American Geologist, 8, 77.
Dang, M.-Z., Rancourt, D.G., Dutrizac, J.E., Lamarche, G. and Provencher, R. (1997) Protohematite-hydrohematite-hematite: structuro-chemical phase relationships in hematite-like materials. Clays Our Future, Proceedings of the 11th International Clay Conference, 265–270.
Drits, V.A., Sakharov, B.A., Salyn, A.L. and Manceau, A. (1993) Structural model for ferrihydrite. Clay Minerals, 28, 185–207.
Drits, V.A., Gorshkov, A.I., Sakharov, B.A., Salyn, A.L., Manceau, A. and Sivtsov, A.B. (1995) Ferrihydrite and its phase transformations during heating in the oxidizing and reducing environments. Lithology and Mineral Resources, 1, 76–84.
Eggleton, R.A. and Fitzpatrick, R.W. (1988) New data and a revised structural model for ferrihydrite. Clays and Clay Minerals, 36, 111–124.
Fischer, W.R. (1988) Microbiological reactions of iron in soils. Pp. 715–748 in: Iron in Soils and Clay Minerals (J.W. Stucki, B.A. Goodman and U. Schwertmann, editors). NATO ASI Series, 217. D. Reidel Publishing Company, Dordrecht, The Netherlands.
Friedman, G.M. and Sanders, J.E. (1978) Principles of Sedimentology. Wiley, New York, NY, p. 235.
Glasauer, S.M., Hug, P., Weidler, P.G. and Gehring, A.U. (2000) Inhibition of sintering by Si during the conversion of Si-rich ferrihydrite to hematite. Clays and Clay Minerals, 48, 51–56.
Inouye, K., Ishii, S., Kaneko, K. and Ishikawa, T. (1972) Effect of copper(II) on the crystallization of α-iron oxide hydrate [α-FeOOH]. Zeitschrift für Anorgische und Allgemeine Chemie, 391, 86–96.
Jambor, J.L. and Dutrizac, J.E. (1998) Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chemical Reviews, 98, 2549–2585.
Kündig, W. and Bömmel, H. (1966) Some properties of supported small a-Fe2O3 particles determined with the Mössbauer effect. Physical Review, 142, 327–333.
Labuda, E. (1996) Reactions of iron oxides at elevated pressures and temperatures. PhD dissertation, The Catholic University of America, Washington, D.C., 262 pp.
Lewis, D.G. and Schwertmann, U. (1979) The influence of aluminum on the formation of iron oxides. IV. The influence of aluminum concentration, hydroxide concentration, and temperature. Clays and Clay Minerals, 27, 195–200.
Lunden, D.G. and Dean, W. (1979) Biochemistry of iron. Pp. 211–251 in: Biogeochemical Cycling of Mineral-forming Elements (P.A. Trudinger and D.J. Swaine, editors). Elsevier, Amsterdam.
Pfaff, G. and Feltz, A. (1990) Solid-state reactivity and mechanisms in oxide systems. VI. Sintering behaviour of hematite prepared by the hydrothermal method. Solid State Ionics, 38, 25–29.
Ruan, H.D., Frost, R.L., Kloprogge, J.T. and Duong, L. (2002) Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 58A, 967–981.
Russell, J.D. (1979) Infrared spectroscopy of ferrihydrite: Evidence for the presence of structural hydroxyl groups. Clay Minerals, 14, 109–114.
Schultz, M.F., Benjamin, M.M. and Ferguson, J.F. (1987) Adsorption and desorption of metals on ferrihydrite: Reversibility of the reaction and sorption properties of the regenerated solid. Environmental Science and Technology, 21, 863–869.
Schwertmann, U. and Cornell, R.M. (2000) Iron Oxides in the Laboratory, 2nd edition. Wiley-VCH, Weinheim, Germany.
Schwertmann, U. and Murad, E. (1990) The influence of aluminum on iron oxides: XIV. Aluminum-substituted magnetite synthesized at ambient temperatures. Clays and Clay Minerals, 38, 196–202.
Schwertmann, U., Fitzpatrick, W.R., Taylor, R.M. and Lewis, D.G. (1979) The influence of aluminum on iron oxides. Part II. Preparation and properties of aluminum-substituted hematites. Clays and Clay Minerals, 27, 105–112.
Schwertmann, U., Friedl, J. and Stanjek, H. (1999) From Fe(III) ions to ferrihydrite and then to hematite. Journal of Colloid and Interface Science, 209, 215–223.
Stanjek, H. and Weidler, P.G. (1992) The effect of dry heating on the chemistry, surface area, and oxalate solubility of synthetic 2-line and 6-line ferrihydrites. Clay Minerals, 27, 397–412.
Torrent, J., Guzman, R. and Parra, M.A. (1982) Influence of relative humidity on the crystallization of Fe(III) oxides from ferrihydrite. Clays and Clay Minerals, 30, 337–340.
Turner, P. (1980) Continental Red Beds. Developments in Sedimentology, Vol. 29. Elsevier, Amsterdam.
Towe, K.M. and Bradley, W.F (1967) Mineralogical constitution of colloidal hydrous ferric oxides. Journal of Colloid and Interface Science, 24, 384–392.
Van Houten, F.B. (1972) Iron and clay in tropical savanna alluvium, Northern Colombia. Contribution to the Origin of Red Beds. Geological Society of America Bulletin, 83, 2761–2772.
Van Houten, F.B. (1973) Origin of Red Beds. A review. 1961–1972. Annual Review of Earth and Planetary Science, 1, 39–61.
Varrin, R.D., Jr. (1996) Characterization of PWR Steam Generator Deposits, EPRI TR-106048. Electric Power Research Institute, Palo Alto, California, USA.
Vempati, R.K. and Loeppert, R.H. (1989) Influence of structural and absorbed Si on the transformation of synthetic ferrihydrite. Clays and Clay Minerals, 37, 273–279.
Walker, T.R. (1967) Formation of Red Beds in modern and ancient deserts. Ganz-mavag Bulletin 78, 353–368.
Walker, T.R. (1974) Formation of Red Beds in moist tropical climates. Hypothesis. Geological Society of America Bulletin, 85, 633–638.
Weidler, P.G. (1995) Surfaces of iron oxides. Dissertation, Technical University of Munich, Germany, 108 pp.
Wightman, P.G. (2002) New insights into bacteria-related geochemical processes: co-adsorption, temperature dependence of protonation, and effects on iron-hydroxide precipitation. Dissertation, University of Notre Dame, Indiana, USA, 104 pp.
Wolska, E. (1977) The significance of aluminum traces in the elimination of the goethite phase during the ageing of amorphous iron(III) hydroxide. Monatshefte für Chemie, 108, 819–828.
Wolska, E. (1981) The structure of hydrohematite. Zeitschrift für Kristallographie, 154, 69–75.
Wolska, E. and Schwertmann, U. (1989) Nonstoichiometric structures during dehydroxylation of goethite. Zeitschrift für Kristallographie, 189, 223–237.
Zhao, J., Huggins, F.E., Feng, Z. and Huffman, G.P. (1994) Ferrihydrite: surface structure and its effect on phase transformation. Clays and Clay Minerals, 42, 737–746.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labuda, E., Cherepakhov, G. & Barkatt, A. Formation of hard hematite-cemented solids in steam generators: An analog of lithification of Fe-containing sedimentary rocks. Clays Clay Miner. 55, 59–70 (2007). https://doi.org/10.1346/CCMN.2007.0550105
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2007.0550105