Abstract
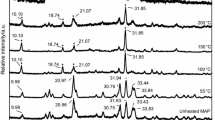
Mineralogical and thermal characteristics of synthetic Al-, Cr-, Mn-, Ni- and Ti-bearing goethites, synthesized via alkaline hydrolysis of metal-ferrihydrite gels, were investigated by powder X-ray diffraction and differential thermal analysis. Shifts in unit-cell dimensions were consistent with size of substituent metal ions and confirmed the incorporation of Al3+, Cr3+, Mn3+, Ni2+ and Ti4+ in the goethite structure. A weight loss of 6.2 wt.% for goethite containing 12.2 mol.% Ti, being significantly less than for stoichiometric goethite, is consistent with the replacement of Fe by Ti in the goethite structure coupled with the substitution of O2− ions for OH− (i.e. proton loss). These data provide the first confirmation of the direct replacement of Fe by Ti within goethite. Formation of multiple dehydroxylation endotherms for goethite containing 4.5 mol.% Al, 15.3 mol.% Mn and 12.2 mol.% Ti was not attributed to the decomposition of surface OH groups or related simply to the crystallinity of precursor goethite (‘high-a’ vs. ‘low-a’) as defined by the magnitude of a. Instead, endotherm doublet formation was associated with weight loss due to the dehydroxylation of goethite remaining after initial phase transformation to protohematite and to the evolution of OH− associated with the rapid increase in crystallite size of protohematite directed primarily along the a direction. Development of the first endotherm is due to initial dehydroxylation and transformation to protohematite. With continued heating of well ordered goethite or goethite containing moderate to high levels of substituent cations, domain growth along the a direction is delayed or inhibited to a critical point that provides enough thermal energy to enable goethite transformation to proceed to completion and for proto-hematite domain growth to occur. This results in the formation of a second endotherm. For less well ordered goethite and/or goethite containing only low levels of foreign metal cations, protohematite domain growth is not inhibited and proceeds continuously with heating to give only a single endotherm.
Similar content being viewed by others
References
Anand, R.R. and Gilkes, R.J. (1987) The association of maghemite and corundum in Darling Range latentes, Western Australia. Australian Journal of Soil Research, 25, 303–311.
Bernai, J.D., Dasgupta, D.R. and Mackay, A.L. (1959) The oxides and hydroxides of iron and their structural interrelationships. Clay Minerals Bulletin, 4, 15–30.
Brown, G. (1980) Associated minerals. Pp. 361–410 in: Crystal Structures of Clay Minerals and their X-ray Identification (G.W. Brindley and G. Brown, editors). Monograph 5, Mineralogical Society, London.
Burns, R.G. (1970) Mineralogical Applications of Crystal Field Theory. Cambridge University Press, London, 1st edition.
Carvalho-de-Silva, M.L., Ramos, A.Y., Tolentino, H.C.N., Enweiler, J., Netto, S.M. and Alves, M.C.M. (2003) Incorporation of Ni into natural goethite: An investigation by X-ray absorption spectroscopy. American Mineralogist, 88, 876–882.
Cornell, R.M. and Schneider, W. (1989) Formation of goethite from ferrihydrite at physiological pH under the influence of cysteine. Polyhedron, 8, 149–155.
Cornell, R.M., Giovanoli, R. and Schneider, W. (1992) The effect of nickel on the conversion of amorphous iron (III) hydroxide into more crystalline iron oxides in alkaline media. Journal of Chemistry and Technical Biotechnology, 53, 73–79.
Cornell, R.M., Mann, S. and Skarnulis, A.J. (1983) A high resolution electron microscopy examination of domain boundaries in crystals of synthetic goethite. Journal of the Chemical Society — Faraday Transactions, 1, 2679–2684.
Derie, R., Ghodsi, M. and Calvo-Roche, C. (1976) DTA study of the dehydration of synthetic goethite α-FeOOH. Journal of Thermal Analysis, 9, 435–440.
Fitzpatrick, R.W. and Chittleborough, D.J. (2002) Titanium and zirconium minerals. Pp. 667–690 in: Soil Mineralogy with Environmental Applications (J.B. Dixon and D.G. Schulze, editors). SSSA Book Series No 7, Soil Science Society of America, Madison, Wisconsin.
Fitzpatrick, R.W., LeRoux, J. and Schwertmann, U. (1978) Amorphous and crystalline titanium and iron-titanium oxides in synthetic preparations, at near ambient conditions, and in soil clays. Clays and Clay Minerals, 26, 189–201.
Ford, R.G. and Bertsch, P.M. (1999) Distinguishing between surface and bulk dehydration-dehydroxylation reactions in synthetic goethites by high-resolution thermogravimetric analysis. Clays and Clay Minerals, 47, 329–337.
Francombe, M.H. and Rooksby, H.P. (1959) Structure transformations affected by the dehydration of diaspore, goethite and delta ferric oxide. Clay Minerals Bulletin, 4, 1–14.
Gasser, U.G., Jeanroy, E., Mustin, C., Barres, O., Nuesch, R., Berthelin, J. and Herbillon, A.J. (1996) Properties of synthetic goethites with Co for Fe substitution. Clay Minerals, 22, 31–39.
Goss, C.J. (1987) The kinetics and reaction mechanism of the goethite to hematite transformation. Mineralogical Magazine, 51, 437–451.
Gualtieri, A.F. and Venturelli, P. (1999) In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. American Mineralogist, 84, 895–904.
Handbook of Chemistry and Physics (1988) Table 1. Bond strengths in diatomic molecules. R. Weast (editor), CRC Press Inc., Florida.
Hsu, P.H. (1989) Aluminum hydroxides and oxyhydroxides. Pp. 99–143 in: Minerals in Soil Environments (J.B. Dixon and S.B. Weed editors). Soil Science Society of America, Madison, Wisconsin.
Huynh, T., Tong, A.R., Singh, B. and Kennedy, B.J. (2003) Cd-substituted goethites — a structural investigation by synchrotron X-ray diffraction. Clays and Clay Minerals, 51, 397–402.
JCPDS (1988) Mineral Powder Diffraction File, Data Book. International Centre for Diffraction Data, Joint Committee on Powder Diffraction Standards, JCPDS, Pennsylvania, USA.
Klug, H.P. and Alexander, L.E. (1974) X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials. John Wiley and Sons, New York, 996 pp.
Mackenzie, R.C. and Berggren, G. (1970) Oxides and oxyhydroxides of higher valency elements. Pp. 272–302: Differential Thermal Analysis (R.C. Mackenzie editor). Academic Press, London.
Mackenzie, R.C. and Mitchell, B.D. (1970) Technique. Pp. 101–122 in: Differential Thermal Analysis (R.C. Makenzie, editor). Academic Press, London.
Mackenzie, R.C., Paterson, E. and Swaffield, R. (1981) Observation of surface characteristics by DSC and DTA. Journal of Thermal Analysis, 22, 269–274.
Manceau, A., Schlegel, M.L., Musso, M., Sole, V.A., Gauthier, C., Petit, P.E. and Trolard, F. (2000) Crystal chemistry of trace elements in natural and synthetic goethite. Geochimica et Cosmochimica Acta, 64, 3643–3661.
Morris, R.V. and Lauer, Jr. H.V. (1981) Stability of goethite (α-FeOOH) and lepidocrocite (γ-FeOOH) to dehydration by UV radiation: Implications for their occurrence on the Martian surface. Journal of Geophysical Research, 86, 10893–10899.
Naono, H. and Fujiwara, R. (1980) Micropore formation due to thermal decomposition of acicular microcrystals of α-FeOOH. Journal of Colloid and Interface Science, 73, 406–415.
Novak, G.A. and Colville, A.A. (1989) A practical interactive least squares cell-parameter program using an electronic spreadsheet and a personal computer. American Mineralogist, 74, 488–490.
Özdemir, Ö. and Dunlop, D.J. (2000) Intermediate magnetite formation during dehydration of goethite. Earth and Planetary Science Letters, 177, 59–67.
Parfitt, R.L. (1989) Optimum conditions for extraction of Al, Fe and Si from soils with acid oxalate. Communications in Soil Science and Plant Analysis, 20, 801–816.
Parfitt, R.L., Farmer, V.C. and Russell, J.D. (1977) Adsorption on hydrous oxides. I. Oxalate and benzoate on goethite. Journal of Soil Science, 28, 29–39.
Paterson, E. and Swaffield, R. (1980) Influence of adsorbed anions on the dehydroxylation of synthetic goethite. Journal of Thermal Analysis, 18, 161–167.
Ruan, H.D. and Gilkes, R.J. (1995) Dehydroxylation of aluminous goethite: Unit cell dimensions, crystal size and surface area. Clays and Clay Minerals, 43, 196–211.
Scheinost, A.C., Stanjek, H., Schulze, D.G., Gasser, U. and Sparks, D.L. (2001) Structural environment and oxidation state of Mn in goethite-groutite solid-solutions. American Mineralogist, 86, 139–146.
Schulze, D.G. and Schwertmann, U. (1984) The influence of aluminium on iron oxides. X. Properties of Al-substituted goethite. Clay Minerals, 19, 521–539.
Schulze, D.G. and Schwertmann, U. (1987) The influence of aluminium on iron oxides. XIII. Properties of goethites synthesized in 0.3 M KOH at 25°C. Clay Minerals, 22, 83–92.
Schwertmann, U. (1984) The double dehydroxylation peak of goethite. Thermochimica Acta, 78, 39–46.
Schwertmann, U. and Cornell, R.M. (1991) Iron Oxides in the Laboratory. VCH Publishing, Weinheim, Germany, 137 pp.
Schwertmann, U. and Pfab, G. (1994) Structural vanadium in synthetic goethite. Geochimica et Cosmochimica Acta, 58, 4349–4352.
Schwertmann, U. and Taylor, R.M. (1989) Iron Oxides. Pp. 379–438 in: Minerals in Soil Environments (J.B. Dixon and S.B. Weed, editors). Soil Science Society of America, Madison Wisconsin, USA.
Schwertmann, U., Cambier, P. and Murad, E. (1985) Properties of goethites of varying crystallinity. Clays and Clay Minerals, 33, 369–378.
Schwertmann, U., Gasser, U. and Sticher, H. (1989) Chromium-for-Fe substitution in synthetic goethites. Geochimica et Cosmochimica Acta, 53, 1293–1297.
Shannon, R.D. (1976) Revised effective ionic radii and systematic studies of inter-atomic distances in halides and chalcogenides. Acta Crystallographica, A32, 751–767.
Singh, B. and Gilkes, R.J. (1992) Properties and distribution of iron oxides and their association with minor elements in the soils of south-western Australia. Journal of Soil Science, 43, 77–93.
Smith, K.L. and Eggleton, R.A. (1983) Microstructures of botryoidal goethites. Clays and Clay Minerals, 31, 392–396.
Stiers, W. and Schwertmann, U. (1985) Evidence for manganese substitution in synthetic goethite. Geochimica el Cosmochimica Acta, 49, 1909–1911.
Suter, D., Banwart, S. and Stumm, W. (1991) Dissolution of hydrous iron(III) oxides by reductive mechanisms. Langmuir, 7, 809–813.
Tessens, E. and Zauyah, S. (1982) Positive permanent charge in Oxisols. Soil Science Society of America Journal, 46, 1103–1106.
Trolard, F., Bourrie, G., Jeanroy, E., Herbillon, A.J. and Martin, H. (1995) Trace metals in natural iron oxides from latentes: A study using selective kinetic extraction. Geochimica et Cosmochimica Acta, 59, 1285–1297.
van Oosterhout, G.W. and Rooijmans, C.J.M. (1958) A new superstructure in gamma ferric oxide. Nature, 181, 44–45.
Vempati, R.K., Morris, R.V., Lauer, H.V. Jr. and DeHart, J. (1991) Synthesis and properties of Ti-substituted goethites and hematites. 28th Annual Clay Minerals Society, Houston, Texas.
Watari, F., Delavignette, P., Van Lunduyt, J. and Amelinckx, S. (1983) Electron microscopic study of dehydration transformations: The formation of ‘superstructures’ on the dehydration of goethite and diaspore. Journal of Solid State Chemistry, 29, 417–427.
Weissenborn, P.K., Dunn, J.G. and Warren, L.J. (1994) Quantitative thermogravimetric analysis of haematite, goethite and kaolinite in Western Australian iron ores. Thermochimica Acta, 239, 147–156.
Wells, M.A. (1998) Mineral, chemical and magnetic properties of synthetic, metal-substituted goethite and hematite. PhD thesis, University of Western Australia, 401 pp.
Wolska, E. and Schwertmann, U. (1989) Nonstoichiometric structures during dehydroxylation of goethite. Zeitschrift für Kristallographie, 189, 223–237.
Wolska, E. and Szajda, W. (1985) Structural and spectroscopic characteristics of synthetic hydrohematite. Journal of Materials Science, 20, 4407–4412.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wells, M.A., Fitzpatrick, R.W. & Gilkes, R.J. Thermal and Mineral Properties of Al-, Cr-, Mn-, Ni- and Ti-Substituted Goethite. Clays Clay Miner. 54, 176–194 (2006). https://doi.org/10.1346/CCMN.2006.0540204
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2006.0540204