Abstract
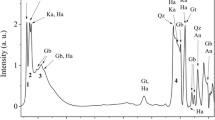
Eighteen purified kaolin samples from Thai Ultisols were studied by X-ray diffraction, X-ray fluorescence, transmission electron microscopy and BET methods. Minor amounts of inhibited vermiculite, quartz and anatase were general contaminants of the kaolins which had an average chemical composition of 403 g kg−1 Al2O3, 550 g kg−1 SiO2, 25.3 g kg−1 Fe2O3, 15.6 g kg−1 TiO2 and 4.65 g kg−1 K2O on an ignited basis. Appreciable concentrations of Mn, Co, Ni, Cu, Zn, As and Pb were present and most of the Ni, Cu and Zn in the original clay fraction was retained in the deferrated kaolin concentrate. It was not possible to determine if these elements are present as structural ions in kaolin crystals.
The kaolins exhibited a variety of crystal morphologies ranging from sub-micron, euhedral, hexagonal plates to anhedral plates and tubes. Their specific surface areas ranged from 15.9 to 61.4 m2g−1 (mean 44.9 m2g−1) and surface area increased with decrease in crystal size. The cation exchange capacity of the kaolins ranged from 7.2 to 23.4 cmolc kg−1 and surface charge density from 0.16 to 0.99 C m−2 but these values are sensitive to the presence of contaminants. Structural iron ranged from 12.4 to 44.8 g kg−1 Fe2O3 and there was an increase in structural defects towards the soil surface associated with an increase in the amount of structural iron.
Similar content being viewed by others
References
Aylmore, L.A.G., Sills, I.D. and Quirk, J.P. (1970) Surface area of homoionic illite and montmorillonite clay minerals as measured by sorption of nitrogen and carbon dioxide. Clays and Clay Minerals, 18, 91–96.
Bolland, M.D.A., Posner, A.M. and Quirk, J.P. (1976) Surface charge on kaolinites in aqueous suspension. Australian Journal of Soil Research, 14, 197–216.
Brady, N.C. and Ray, R.W. (2002) The Nature and Properties of Soils, 13th edition. MacMillan Co., New York, 960 pp.
Brindley, G.W., Kao, C.C., Harrison, J.L., Lipsicas, M. and Raythatha, R. (1986) Relation between structure disorder and other characteristics of kaolinites and dickites. Clays and Clay Minerals, 34, 239–249.
Brown, G. and Brindley, G.W. (1980) X-ray diffraction procedures for clay mineral identification. Pp. 305–359 in: Crystal Structures of Clay Minerals and their X-ray Identification (G.W. Brindley and G. Brown, editors). Monograph 5, Mineralogical Society, London.
Cases, J.M., Cunin, P., Grillet, Y., Poinsignon, C. and Yvon, J. (1986) Methods of analyzing the morphology of kaolinites: relations between crystallographic and morphological properties. Clay Minerals, 21, 55–68.
De Alwis, K.A. and Pluth, D.J. (1976) The red latosols of Sri Lanka: I. Macro-morphological, physical and chemical properties, genesis and classification. II. Mineralogy and weathering. Soil Science Society of America Journal, 40, 912–928.
Dixon, J.B. (1989) Kaolin and serpentine group minerals. Pp. 467–525 in: Minerals in Soil Environments, 2nd edition (J.B. Dixon and S.B. Weed, editors). SSSA Book Series 1, Soil Science Society of America, Madison, Wisconsin.
Ferris, A.P. and Jepson, W.B. (1975) The exchange capacity of kaolinite and the preparation of homoionic clays. Journal of Colloid and Interface Science, 51, 245–259.
Gee, G.W. and Bauder, J.W. (1986) Particle-size analysis. Pp. 383–411 in: Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods (A. Klute, editor). Monograph 9. American Society of Agronomy, Madison, Wisconsin.
Gilkes, R.J. and Suddhiprakam, A. (1979) Biotite alteration in deeply weathered granite. I. Morphological, mineralogical and chemical properties. Clays and Clay Minerals, 27, 349–360.
Hart, R.D., Gilkes, R.J., Siradz, S. and Singh, B. (2002) The nature of soil kaolins from Indonesia and Western Australia. Clays and Clay Minerals, 50, 198–207.
Hart, R.D., Wiriyakitnateekul, W. and Gilkes, R.J. (2003) Properties of kaolins from Thailand. Clay Minerals, 39, 71–94.
Hughes, J.C. and Brown, G. (1979) A crystallinity index for soil kaolins and its relation to parent rock, climate and soil maturity. Journal of Soil Science, 30, 557–563.
Jungerius, P.D. and Levelt, T.W.M. (1964) Clay mineralogy of soils over sedimentary rock in Eastern Nigeria. Soil Science, 97, 89–95.
Juo, A.S.R. (1980) Mineralogical characteristics of Alfisols and Ultisols. Pp. 69–86 in: Soils with Variable Charge (B.K.G. Theng, editor). New Zealand Society of Soil Science, Lower Hutt, New Zealand.
Kheoruenromne, I. and Kesawapitak, P. (1989) Management of acid soils for food crop production in Thailand. Pp. 100–109 in: Management of Acid Soils in the Humid Tropics in Asia (E.T. Craswell and E. Pushparajah, editors). ACIAR Monograph 13 (IBSRAM 1).
Kheoruenromne, I. (1999) Soil Survey Laboratory Manual. Department of Soil Science, Faculty of Agriculture, Kasetsart University, Bangkok, 182 pp. (in Thai).
Klug, H.P. and Alexander, L.E. (1954) X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd edition. John Wiley and Sons Inc., New York, 966 pp.
Koppi, A.J. and Skjemstad, J.O. (1981) Soil kaolins and their genetic relationships in southeast Queensland, Australia. Journal of Soil Science, 32, 661–672.
Ma, C. and Eggleton, R.A. (1999) Cation exchange capacity of kaolinite. Clays and Clay Minerals, 47, 174–180.
McCrea, A.F., Anand, R.R. and Gilkes, R.J. (1990) Mineralogical and physical properties of lateritic pallid zone materials developed from granite and dolerite. Geoderma, 47, 33–57.
MacEwan, D.M.C. and Wilson, M.J. (1980) Interlayer and intercalation complexes of clay minerals. Pp. 197–248 in: Crystal Structures of Clay Minerals and their X-ray Identification (G.W. Brindley and G. Brown, editors). Monograph 5, Mineralogical Society, London.
Mehra, O.P. and Jackson, M.L. (1960) Iron oxide removal from soils and clay hy a dithionite-citrate system buffered with sodium hicarhonate. Clays and Clay Minerals, 1, 317–327.
Melo, V.F., Singh, B., Schaefer, C.E.G.R., Novais, R.F. and Fontes, M.P.F. (2001) Chemical and mineralogical properties of kaolinite-rich Brazilian soils. Soil Science Society of America Journal, 65, 1324–1333.
Mestdagh, M.M., Vielvoye, L. and Herhillon, A.J. (1980) Iron in kaolinite: II. The relationship between kaolinite crystallinity and iron content. Clay Minerals, 15, 1–13.
Montes, C.R., Melfi, A.J., Carvalho, A., Vieira-Coelho, A.C. and Formoso, M.L.L. (2002) Genesis, mineralogy and geochemistry of kaolin deposits of the Jari River, Amapa State, Brazil. Clays and Clay Minerals, 50, 494–503.
Norrish, K. and Chappell, B.W. (1977) X-ray fluorescence spectrometry. Pp. 201–272 in: Physical Methods in Determinative Mineralogy (J. Zussman, editor). Academic Press, Inc., New York.
Rayment, G.E. and Higginson, E.R. (1992) Australian Laboratory Handbook of Soil and Water Chemical Methods. Australian Soil and Land Survey Handbook, Inkata Press, Melbourne.
Schwertmann, U. and Herhillon, A.J. (1992) Some aspects of fertility associated with the mineralogy of highly weathered tropical soils. Pp. 47–59 in: Myths and Science of Soils of the Tropics (R. Lai, editor). Special Publication 29, Soil Science Society of America, Madison, Wisconsin.
Singh, B. and Gilkes, R.J. (1992a) Weathering of a chromium muscovite to kaolinite. Clays and Clay Minerals, 40, 212–229.
Singh, B. and Gilkes, R.J. (1992b) XPAS: An interactive program to analyse X-ray powder diffraction patterns. Powder Diffraction, 7, 6–10.
Singh, B. and Gilkes, R.J. (1992c) Properties of soil kaolinite from south-western Australia. Journal of Soil Science, 43, 645–666.
Soil Survey Division Staff (1993) Soil Survey Manual. Soil Conservation Service, United States Department of Agriculture Handbook 18, Washington, D.C.
Soil Survey Staff (1999) Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd edition, United States Department of Agriculture, US Government Printing Office, Washington, D.C.
Soil Survey Staff (2003) Keys to Soil Taxonomy, 9th edition, United States Department of Agriculture. US Government Printing Office, Washington, D.C., 332 pp.
Stace, H.C.T., Hubble, G.D., Brewer, R., Northcote, K.H., Sleeman, J.R., Mulcahy, M.J. and Hallsworth, E.G. (1965) A Handbook of Australian Soils. Rellim Technical Publications, Adelaide, South Australia, 425 pp.
Suraj, G., Iyer, C.S.P., Rugmini, S. and Lalithamhika, M. (1997) The effects of micronization on kaolinites and their sorption behavior. Applied Clay Science, 12, 111–130.
Tari, G., Bobos, I., Gomes, C.S.F. and Ferreira, J.M.F. (1999) Modification of surface charge properties during kaolinite to halloysite-7 Å transformation. Journal of Colloid and Interface Science, 210, 360–366.
Thomas, G.W. (1996) Soil pH and soil acidity. Pp. 475–490 in: Methods of Soil Analysis Part 3: Chemical Methods (D.L. Sparks, A.L. Page, P.A. Helmke, R.H. Loeppert, P.N. Soltanpour, M.A. Tahatahai, C.T. Johnston and M.E. Sumner, editors). American Society of Agronomy, Madison, Wisconsin.
Trunz, V. (1976) The influence of crystallite size on the apparent basal spacings of kaolinite. Clays and Clay Minerals, 24, 84–87.
Webster, R. (1965) A catena of soils on the Northern Rhodesia Plateau. Journal of Soil Science, 16, 31–43.
Yong, R.N., Mohamed, A.M.O. and Warketin, B.P. (1992) Principles of Contaminant Transport in Soil. Elsevier, Amsterdam, 327 pp.
Yoothong, K., Monchareon, L., Vijamson, P. and Eswaran, H. (1997) Clay mineralogy in Thai soils. Applied Clay Science, 11, 357–371.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanket, W., Suddhiprakarn, A., Kheoruenromne, I. et al. Chemical and Crystallographic Properties of Kaolin from Ultisols in Thailand. Clays Clay Miner. 53, 478–489 (2005). https://doi.org/10.1346/CCMN.2005.0530505
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2005.0530505