Abstract
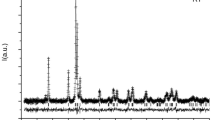
Ferrihydrite is a poorly crystalline Fe oxide of which 2-(XRD)line and 6-line varieties are commonly used in experiments, although species with intermediate numbers of peaks have been found in nature. To simulate nature, we synthesized two continuous series of ferrihydrites with between 2 and 6–7 peaks at room temperature in two different ways: (1) by varying the rate of hydrolysis of an Fe(NO3)3 solution (HR series); and (2) by oxidizing an FeCl2 solution containing up to 73 mmol Si/L (Si series), both at pH 7. Mössbauer spectra at 4.2 K showed that the ferrihydrites of the HR series had a constant magnetic hyperfine field (Bhf) at 4.2 K of 48.8 T whereas Bhf in the Si series dropped from 49.4 to 46.7 T as the Si content of the ferrihydrites increased from 0 to 74.7 g/kg of Si. Temperature scans between 4 and 170 K illustrate that the magnetic order breaks down at a temperature which is lower the higher the hydrolysis rate and the Si concentration in the ferrihydrite.
Similar content being viewed by others
References
Carlson, L. and Schwertmann, U. (1981) Natural ferrihydrites in surface deposits from Finland and their association with silica. Geochimimica et Cosmochimica Acta, 45, 421–429.
Carlson, L. and Schwertmann, U. (1990) The effect of CO2 and oxidation rate on the formation of goethite versus lepidocrocite at pH 6 and 7. Clay Minerals, 25, 65–71.
Chukhrov, F.V., Zvyagin, B.B., Ermilova, L.P. and Gorshkov, A.I. (1973a) New data on iron oxides in the weathering zone. Proceedings of the International Clay Conference, Madrid (1972), 333–341.
Cornell, R.M. and Schwertmann, U. (2003) The Iron Oxides, 2nd edition. Wiley-VCH, Weinheim, Germany, 664 pp.
Drits, V.A., Sakharov, B.A., Salyn, A.L. and Manceau, A. (1993) Structural model for ferrihydrite. Clay Minerals, 28, 185–207.
Dzombak, D.A. and Morel, F.M.M. (1990) Surface Complexation Modeling. Hydrous Ferric Oxide. J. Wiley, New York, 393 pp.
Eggleton, R.A. and Fitzpatrick, R.W. (1988) New data and a revised structural model for ferrihydrite. Clays and Clay Minerals, 36, 111–124.
Friedl, J. and Schwertmann, U. (1996) Aluminium influence on iron oxides: XVIII. The effect of Al substitution and crystal size on magnetic hyperfine fields of natural goethites. Clay Minerals, 31, 455–464.
Jambor, J.L. and Dutrizac, J.E. (1998) Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chemical Reviews, 98, 2549–2585.
Janney, D.E., Cowley, J.M. and Buseck P.R. (2000a) Transmission electron microscopy of synthetic 2- and 6-line ferrihydrite. Clays and Clay Minerals, 48, 111–119.
Janney, D.E., Cowley, J.M. and Buseck P.R. (2000b) Structure of synthetic 2-line ferrihydrite by electron nanodiffraction, American Mineralogist, 85, 1180–1187.
Kampf, N. and Schwertmann, U. (1983) Goethite and hematite in a climosequence in Southern Brazil and their application in classification of kaolinitic soils. Geoderma, 29, 27–39.
Lewis, D.G. and Cardile, C.M. (1989) Hydrolysis of Fe(III) solution to hydrous iron oxides. Australian Journal of Soil Research, 27, 103–115.
Murad, E. (1996) Magnetic properties of microcrystalline iron(III) oxides and related materials as reflected in their Mössbauer spectra. Physics and Chemistry of Minerals, 23, 248–262.
Murad, E. and Schwertmann, U. (1980) The Mössbauer spectrum of ferrihydrite and its relation to those of other iron oxides. American Mineralogist, 65, 1044–1049.
Murad, E., Bowen, L.H., Long, G.J. and Quin, T.G. (1988) The influence of crystallinity on the magnetic ordering in natural ferrihydrites. Clay Minerals, 23, 161–173.
Murphy, P.J., Posner, A.M. and Quirk, J.P. (1976) Characterization of partially neutralized ferric nitrate solutions. Journal of Colloid and Interface Science, 56, 270–283.
Schwertmann, U. and Cornell, R.M. (2000) Iron Oxides in the Laboratory, 2nd edition. VCH, Weinheim, Germany, 188 pp.
Schwertmann, U. and Taylor, R.T. (1979) Natural and synthetic poorly crystalline lepidocrocite. Clay Minerals, 14, 285–293.
Stanjek, H. and Schwertmann, U. (1992) The influence of aluminum on iron oxides. Part XVI: Hydroxyl and aluminum substitution in synthetic hematites. Clays and Clay Minerals, 40, 347–354.
Towe, K.M. and Bradley, W.F. (1967) Mineralogical constitution of colloidal “hydrous ferric oxides”. Journal of Colloid and Interface Science, 24, 384–392.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwertmann, U., Friedl, J. & Kyek, A. Formation and Properties of a Continuous Crystallinity Series of Synthetic Ferrihydrites (2- to 6-line) and their Relation to FeOOH Forms. Clays Clay Miner. 52, 221–226 (2004). https://doi.org/10.1346/CCMN.2004.0520208
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2004.0520208