Abstract
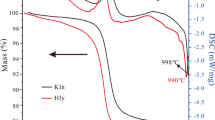
Palygorskite-indigo and sepiolite-indigo adducts (2 wt.% indigo) were prepared by crushing the two compounds together in a mortar and heating the resulting mixtures at 150 and 120°C, respectively, for 20 h. The samples were tested chemically to ensure that they displayed the characteristic properties of Maya Blue. Textural analysis revealed that no apparent changes in microporosity occurred in sepiolite or palygorskite after thermal treatment at 120°C (sepiolite) and 150°C (palygorskite) for 20 h. Micropore measurements showed a loss of microporosity in both sepiolite and palygorskite after reaction with indigo. The TGA-DTG curves of the sepiolite-indigo and palygorskite-indigo adducts were similar to their pure clay mineral counterparts except for an additional weight loss at ∼360°C due to indigo.
The 29Si CP/MAS-NMR spectrum of the heated sepiolite-indigo adduct is very reminiscent of the spectrum of dehydrated sepiolite. Crushing indigo and sepiolite together initiates a complexation, clearly seen in the 13C CP/MAS-NMR spectrum, which can be driven to completion by heat application. In contrast to the broad peaks of the pure indigo 13C CP/MAS-NMR spectrum, the sepiolite-indigo adduct spectrum consists of a well-defined series of six narrow peaks in the 120.0–125.0 ppm range. In addition, the sepiolite-indigo spectrum has two narrow, shifted peaks corresponding to the carbonyl group and the C-7 (C-16) of indigo. A model is proposed in which indigo molecules are rigidly fixed to the clay mineral surface through hydrogen bonds with edge silanol groups, and these molecules act to block the nano-tunnel entrances.
Similar content being viewed by others
References
Ahlrichs, J.L., Serna, C. and Serratosa, J.M. (1975) Structural hydroxyls in sepiolite. Clays and Clay Minerals, 23, 119–124.
Brindley, G.W. (1959) X-ray and electron diffraction data for sepiolite. American Mineralogist, 44, 495–500.
Cooksey, C.J. and Dronsfield, A.T. (2002) Adolf von Baeyer and the indigo molecule. Dyes in History and Archaeology, 18, 13–19.
Gettens, R.J. (1962) Maya blue: an unsolved problem in ancient pigments. American Antiquity, 27, 557–564.
Gettens, R.J. and Stout, G.L. (1946) Painting Materials: A Short Encyclopedia. D. Van Nostrand, New York, pp. 130–131.
Gordon, P.F. and Gregory, P. (1983) Indigoid Dyes. Pp. 208–211 in: Organic Chemistry in Colour. Springer-Verlag, Berlin.
Gribova, E.A. (1955) X-ray study of indigo and thioindigo. L. Ya Karpov Physical Chemistry Institute: Doklady Akademii Nauk SSSR, 102, 279–81.
Horvath, G. and Kawazoe, K. (1983) Method for the calculation of effective pore size distribution in molecular sieve carbon. Journal of Chemical Engineering of Japan, 16, 470–475.
Jones, B.F. and Galán, E. (1998) Sepiolite and palygorskite. Pp. 631–674 in: Hydrous Phyllosilicates (S.W. Bailey, editor). Reviews in Mineralogy, 19. Mineralogical Society of America, Washington, D.C.
Jose-Yacaman, M., Rendon, L., Arenas, J. and Puche, M.C.S. (1996) Maya blue paint: an ancient nanostructured material. Science, 273, 223–225.
Kleber, R., Masschelein-Kleiner, L. and Thissen, J. (1967) Study and identification of Maya blue. Studies in Conservation, 12, 41–56.
Kuang, W., Hubbard, B., Moser, A., Facey, G.A. and Detellier, C. (2002) Organo-sepiolite and palygorskite nanocomposites. Proceedings of the 5th International Conference on Solid State Chemistry, Bratislava, Slovak Republic.
Le Van Mao, R., Rutinduka, E., Detellier, C., Gougay, P., Hascoet, V., Tavakoliyan, S., Hoa, S.V. and Matsuura, T. (1999) Mechanical and pore characteristics of zeolite composite membranes. Journal of Materials Chemistry, 9, 783–788.
Merwin, H.E. (1931) In: The Temple of the Warriors at Chichen Itza (E.H. Morris, J. Charlot and A.A. Morris, editors). Carnegie Institution of Washington, Washington, D.C., publ. 406.
Myriam, M., Suarez, M. and Martin-Pozas, J.M. (1998) Structural and textural modifications of palygorskite and sepiolite under acid treatment. Clays and Clay Minerals, 46, 225–231.
Pedro, G. (1972) Report of the AIPEA Nomenclature Committee. AIPEA Newsletter, 4, 3–4.
Polette, L.A., Meitzner, G., Jose-Yacaman, M. and Chianelli, R.R. (2002) Maya blue: application of XAS and HRTEM to materials science in art and archaeology. Microchemical Journal, 71, 167–174.
Ruiz-Hitzky, E. (2001) Molecular access to intracrystalline tunnels of sepiolite. Journal of Materials Chemistry, 11, 86–91.
Serna, C. and van Scoyoc, G.E. (1979) Infrared study of sepiolite and palygorskite surfaces. Pp. 197–206 in: Proceedings of the International Clay Conference, Oxford, 1978 (M.M. Mortland and V.C. Farmer, editors). Elsevier, Amsterdam.
Shepard, A. (1962) Maya blue: alternative hypotheses. American Antiquity, 27, 565–566.
Tagle, A., Paschinger, H., Richard, H. and Infante, G. (1990) Maya blue: its presence in Cuban colonial wall paintings. Studies in Conservation, 35, 156–159.
Van Olphen, H. (1966) Maya Blue: a clay mineral-organic pigment? Science, 154, 645–646.
Wang, Q.K., Matsuura, T., Feng, C.Y., Weir, M.R., Detellier, C., Rutinduka, E. and Le Van Mao, R. (2001) The sepiolite membrane for ultrafiltration. Journal of Membrane Science, 184, 153–163.
Weir, M.R., Facey, G.A. and Detellier, C. (2000) 1H, 2H and 29Si solid state NMR study of guest acetone molecules occupying the zeolitic channels of partially dehydrated sepiolite clay. Studies in Surface Science and Catalysis, 129, 551–558.
Weir, M.R., Rutinduka, E., Detellier, C., Feng, C.Y., Wang, Q., Matsuura, T. and Le Van Mao, R. (2001) Fabrication, characterization and preliminary testing of all-inorganic ultrafiltration membranes composed entirely of a naturally occurring sepiolite clay mineral. Journal of Membrane Science, 182, 41–50.
Weir, M.R., Kuang, W., Facey, G.A. and Detellier, C. (2002) Solid state nuclear magnetic resonance study of sepiolite and partially dehydrated sepiolite. Clays and Clay Minerals, 50, 240–247.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hubbard, B., Kuang, W., Moser, A. et al. Structural Study of Maya Blue: Textural, Thermal and Solid-State Multinuclear Magnetic Resonance Characterization of the Palygorskite-Indigo and Sepiolite-Indigo Adducts. Clays Clay Miner. 51, 318–326 (2003). https://doi.org/10.1346/CCMN.2003.0510308
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2003.0510308