Abstract
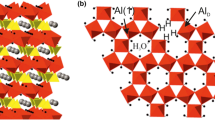
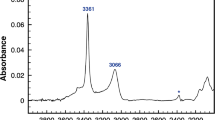
Silanol groups in protonated magadiite (H-magadiite) were characterized by 1H and 2H solid-state nuclear magnetic resonance (NMR). H-magadiite and deuterated (D) magadiite were synthesized by the treatment of Na-rich magadiite with 0.2 N HC1 and 0.2 N DC1, respectively. In the ’H NMR spectrum measured at room temperature, silanol groups of H-magadiite showed two signals at 3.75 and 5.70 ppm, indicating that two types of silanol groups were present. The ratio of silanol groups associated with strong hydrogen bonding (5.70 ppm) to those with weaker hydrogen bonding (3.75 ppm) was 2 to 1. The 2H NMR spectra of deuterated magadiite were measured in the temperature range from 150 to 440 K. In the spectra measured at temperatures below 294 K, silanol groups showed Pake doublet patterns. These patterns were composed of two components corresponding to the two types of silanol groups shown in the ‘H NMR analysis. Both silanol groups produced wobbling motions with increasing temperature. Above 294 K, the profile of the Pake doublet pattern was transformed gradually to a near triangular pattern, indicating that the silanol groups underwent other motions also, such as a two-site jump.
Similar content being viewed by others
References
Almond, G.G., Harris, R.K., and Graham, P. (1994) A study of the layered alkali metal silicate, magadiite, by one- and two-dimensional 1H and 29Si NMR spectroscopy. Journal of the Chemical Society, Chemical Communications, 851–852.
Almond, G.G., Harris, R.K., Franklin, K.R., and Graham, P. (1996) A 23Na NMR study of hydrous layered silicates. Journal of Materials Chemistry, 6, 843–847.
Almond, G.G., Harris, R.K., and Franklin, K.R. (1997) A structural consideration of kanemite, octosilicate, magadiite and kenyaite. Journal of Materials Chemistry, 7, 681–687.
Barnes, R.G. (1974) Deuteron quadrupole coupling tensors in solids. In Advances in Nuclear Quadrupole Resonances, Volume 1, J.A.S. Smith, ed., Heyden, London, 335–355.
Brandt, A., Schwieger, W., and Bergk, K.H. (1987) A new model structure of sheet sodium (Na) silicate hydrates (Na-SH)-theoretical view based on known X-ray and NMR-measurements. Revue de Chimie Minérale, 24, 564–571.
Brandt, A., Schwieger, W., and Bergk, K.H. (1988) Development of a model structure for the sheet silicate hydrates ilerite, magadiite, and kenyaite. Crystal Research and Technology, 23, 1201–1203.
Brindley, G.W. (1969) Unit cell of magadiite in air, in vacuo, and under other conditions. American Mineralogist, 54, 1583–1591.
Butler, L.G. and Brown, T.L. (1981) Nuclear quadrupole coupling constants and hydrogen bonding. A molecular orbital study of oxygen-17 and deuterium field gradients in formaldehyde-water hydrogen bonding. Journal of the American Chemical Society, 103, 6541–6549.
Dailey, J.S. and Pinnavaia, T.J. (1992) Silica-pillared derivatives of H+-magadiite, a crystalline hydrated silica. Chemistry of Materials, 4, 855–863.
Eckert, H., Yesinowski, J.E, and Stolper, E.M. (1989) Quantitative NMR studies of water in silicate glasses. Solid State Ionics, 32/33, 298–313.
Garcés, J.M., Rocke, S.C., Crowder, C.E., and Hasha, D.L. (1988) Hypothetical structures of magadiite and sodium octosilicate and structural relationships between the layered alkali metal silicates and the mordenite- and pentasil-group zeolites. Clays and Clay Minerals, 36, 409–418.
Gluszak, T.J., Chen, D.T., Sharma, S.B., Dumesic, J.A., and Root, T.W. (1992) Observation of Brpnsted acid sites of DY zeolite with deuterium NMR. Chemical Physics Letters, 190, 36–41.
Hadjar, H., Balard, H., and Papirer, E. (1995) Comparison of crystalline (H-magadiite) and amorphous silicas using inverse gas chromatography at finite concentration conditions. Colloids and Surfaces A, 103, 111–117.
Hayashi, S. (1994) Effects of magic-angle spinning on spinlattice relaxations in talc. Solid State Nuclear Magnetic Resonance, 3, 323–330.
Hayashi, S., Ueda, X., Hayamizu, K., and Akiba, E. (1992) NMR study of kaolinite. 1. 29Si, 27Al, and 1H spectra. Journal of Physical Chemistry, 96, 10922–10928.
Hayashi, S., Akiba, E., Miyawaki, R., and Tomura, S. (1994) 2H NMR study of hydrogen bonding in deuterated kaolinite. Clays and Clay Minerals, 42, 561–566.
Hoatson, G.L. and Void, R.L. (1994) 2H-NMR spectroscopy of solids and liquid crystals. In NMR Basic Principles and Progress: Solid State NMR III: Organic Matter, Volume 32, P. Diehl, E. Fluck, H. Günther, R. Kosfeld, and J. Seelig, eds., Spriger-Verlag, Berlin, 1–67.
Huang, Y., Jiang, Z., and Schwieger, W. (1999) Vibrational spectroscopic studies of layered silicates. Chemistry of Materials, 11, 1210–1217.
Kobe, J.M., Gluszak, T.J., Dumestic, J.A., and Root, T.W. (1995) Deuterium NMR characterization of Brpnsted acid sites and silanol species in zeolites. Journal of Physical Chemistry, 99, 5485–5491.
Kosuge, K., Yamazaki, A., Tsunashima, A., and Otsuka, R. (1992) Hydrothermal synthesis of magadiite and kenyaite. Journal of the Ceramic Society of Japan, 100, 326–331.
Lagaly, G., Beneke, K., and Weiss, A. (1975a) Magadiite and H-magadiite: I. Sodium magadiite and some of its derivatives. American Mineralogist, 60, 642–649.
Lagaly, G., Beneke, K., and Weiss, A. (1975b) Magadiite and H-magadiite: II. H-magadiite and its intercalation compounds, American Mineralogist, 60, 650–658.
Landis, M.E., Aufdembrink, B.A., Chu, P., Johnson, I.D., Kirker, G.W., and Rubin, M.K. (1991) Preparation of molecular sieves from dense, layered metal oxides. Journal of the American Chemical Society, 113, 3189–3190.
Mercier, L., Facey, G.A., and Detellier, C. (1994) Organo- layered silicates. Interlamellar intercalation and grafting of ethylene glycol in magadiite. Journal of the Chemical Society, Chemical Communications, 2111–2112.
Ogawa, M., Miyoshi, M., and Kuroda, K. (1998a) Perfluoroalkylsilylation of the interlayer silanol groups of a layered silicate, magadiite. Chemistry of Materials, 10, 3787–3789.
Ogawa, M., Okutomo, S., and Kuroda, K. (1998b) Control of interlayer microstructures of a layered silicate by surface modification with organochlorosilanes. Journal of the American Chemical Society, 120, 7361–7362.
Okutomo, S., Kuroda, K., and Ogawa, M. (1999) Preparation and characterization of silylated-magadiite. Applied Clay Science, 15, 253–264.
Pfeifer, H. (1994) NMR of Solid Surfaces. In NMR Basic Principles and Progress: Solid State NMR II: Inorganic Matter, Volume 31, P. Diehl, E. Fluck, H. Günther, R. Kosfeld, and J. Seelig, eds., Spriger-Verlag, Berlin, 31–90.
Pinnavaia, T.J., Johnson, I.D., and Lipsicas, M. (1986) A 29Si MAS NMR study of tetrahedral site distributions in the layered silicic acid H+-magadiite (H2Si14O29·nH2O) and in Na+-magadiite (Na2Si14O29·nH2O). Journal of Solid State Chemistry, 63, 118–121.
Rojo, J.M., Ruiz-Hitzky, E., Sanz, J., and Serratosa, J.M. (1983) Characterization of surface Si-OH groups in layer silicic acids by IR and NMR spectroscopies. Revue de Chimie Minérale, 20, 807–816.
Rojo, J.M., Ruiz-Hitzky, E., and Sanz, J. (1988) Proton-sodium exchange in magadiite. Spectroscopic study (NMR, IR) of the evolution of interlayer OH groups. Inorganic Chemistry, 27, 2785–2790.
Ruiz-Hitzky, E. and Rojo, J.M. (1980) Intracrystalline grafting on layer silicic acids. Nature, 287, 28–30.
Ruiz-Hitzky, E., Rojo, J.M., and Lagaly, G. (1985) Mechanism of the grafting of organosilanes on mineral surfaces III. Interlamellar grafting of layer silicic acids. Colloid and Polymer Science, 263, 1025–1030.
Satozawa, M., Kunimori, K., and Hayashi, S. (1997) 13C and 1H MAS NMR study of benzene and p-xylene in zeolites and a mesoporous material FSM-16. Bulletin of the Chemical Society of Japan, 70, 97–105.
Scholzen, G., Beneke, K., and Lagaly, G. (1991) Diversity of magadiite. Zeitschrift für Anorganische und Allgemeine Chemie, 597, 183–196.
Schwieger, W., Heidemann, D., and Bergk, K. (1985) Highresolution solid-state silicon-29 nuclear magnetic resonance spectroscopic studies of synthetic sodium silicate hydrates. Revue de Chimie Minérale, 22, 639–650.
Sprung, R., Davis, M.E., Kauffman, J.S., and Dybowski, C. (1990) Pillaring of magadiite with silicate species. Industrial & Engineering Chemistry Research, 29, 213–220.
Wong, S. and Cheng, S. (1993) Preparation and characterization of pillared magadiite. Chemistry of Materials, 5, 770-111.
Xie, X. and Hayashi, S. (1999a) NMR study of kaolinite intercalation compounds with formamide and its derivatives. 1. Structure and orientation of guest molecules. Journal of Physical Chemistry B, 103, 5949–5955.
Xie, X. and Hayashi, S. (1999b) NMR study of kaolinite intercalation compounds with formamide and its derivatives. 2. Dynamics of guest molecules. Journal of Physical Chemistry B, 103, 5956–5962.
Yanagisawa, T., Kuroda, K., and Kato, C. (1988a) Organic derivatives of layered polysilicates I. Xrimethylsilylation of magadiite and kenyaite. Reactivity of Solids, 5, 167–175.
Yanagisawa, T., Kuroda, K., and Kato, C. (1988b) Organic derivatives of layered polysilicates II. Reaction of magadiite and kenyaite with diphenylmethylchlorosilane. Bulletin of the Chemical Society of Japan, 61, 3743–3745.
Yanagisawa, T., Harayama, M., Kuroda, K., and Kato, C. (1990) Organic derivatives of layered polysilicates III. Reaction of magadiite and kenyaite with alkyldimethylchlo- rosilane. Solid State Ionics, 42, 15–19.
Yanagisawa, T., Kuroda, K., Doi, A., and Kato, C. (1991) Heat-treatment of trimethylsilylated layered polysilicates and the variation in their specific surface areas. Clay Science, 8, 107–116.
Yesinowski, J.P and Eckert, H. (1987) Hydrogen environments in calcium phosphates: 1H MAS NMR at high spinning speeds. Journal of the American Chemical Society, 109, 6274–6282.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komori, Y., Miyoshi, M., Hayashi, S. et al. Characterization of Silanol Groups in Protonated Magadiite by 1H and 2H Solid-State Nuclear Magnetic Resonance. Clays Clay Miner. 48, 632–637 (2000). https://doi.org/10.1346/CCMN.2000.0480604
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2000.0480604