Abstract
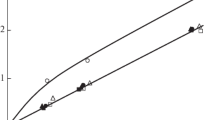
Hydroxyaluminosilicate (HAS) and hydroxyaluminum (HyA) ionic solutions having final Al concentrations ranging from 3.74 to 4.00 mM; NaOH/Al molar ratios of 1.0, 2.0 and 2.5; and Si/Al molar ratios of 0.00, 0.27–0.30, 0.51–0.56 and 0.95–1.01 were prepared through the interaction of AlCl3, or-thosilicic acid and NaOH solutions. When these solutions reacted with <2 µm sized vermiculite (Vt) and montmorillonite (Mt), varying amounts of Al and Si were fixed on Vt and Mt clays. Potassium fixation and exchange capacities of HyA/HAS (OH/Al = 1.0, 2.0 and 2.5)-Vt and HyA/HAS (OH/AI = 2.0)-Mt complexes were compared with those of untreated Vt and Mt at added K levels ranging from 21 to 319 cmolc kg−1. The untreated Vt clay showed K fixation as high as 94 cmolc kg−1, in contrast to only 16 cmolc kg−1 exchangeable K. The untreated Mt fixed a maximum of 9 cmolc K kg−1 out of a total K adsorption capacity of 67 creole kg−1. In the HyA/HAS-Vt complexes, K fixation reduced drastically in comparison to untreated Vt, and ranged from 9 to 24 cmolc kg−1 out of their total K adsorption capacities of 61 to 81 cmolc kg−1. In the HyA/HAS-Mt complexes, too, the amount of K fixed reduced to a great extent in comparison to Mt and ranged from 1.48 to 1.84 cmolc kg−1. Potassium became more exchangeable due to the presence of hydroxy-interlayers in the clays. The reduction in CEC and the well-known propping effects of hydroxy-cations’ islands in the interlayers might have hindered K fixation by the complexes. The relationships of maximum K fixing capacities of the HyA/HAS-Vt complexes with the amounts of Al, Si and Al + Si fixed on Vt were all exponential and negative. However, the amount of Al + Si or only Al fixed on Vt appeared to be the best indicator of K fixation capacities of hydroxyinterlayered Vt clay.
Similar content being viewed by others
References
Akitt JW, Farthing A. 1981. Aluminum-27 nuclear magnetic resonance studies of hydrolysis of aluminum (III). Part 3: Stopped-flow kinetic studies. J Chem Soc Dalton Trans 1981:1609–1614.
Bamhisel RI, Bertsch PM. 1989. Chlorites and hydroxy-interlayered vermiculite and smectite. In: Dixon JB, Weed SB, editors. Minerals in soil environments. Madison, WI: Soil Sci Soc Am. p 729–788.
Barshad I. 1948. Vermiculite and its relation to biotite as revealed by base exchange reactions, X-ray analysis, differential thermal curves and water content. Am Mineral 33:655–678.
Barshad I. 1950. The effect of the interlayer cations on the expansion of mica type crystal lattice. Am Mineral 35:225–238.
Bautista-Tulin AT, Inoue K. 1997. Hydroxy-interlayered minerals in Japanese soils influenced by eolian deposition. Soil Sci Soc Am J 61:631–640.
Bertsch PM, Layton WJ, Barnhisel RI. 1986. Speciation of hydroxy-aluminum solution by wet chemical and aluminum-27 NMR methods. Soil Sci Soc Am J 50:1449–1454.
Bertsch PM, Parker DR. 1996. Aqueous polynuclear aluminum species. In: Sposito G, editor. The environmental chemistry of aluminum. Tokyo: Lewis Publ, CRC Pr. p 117–168.
Bottero JY, Cases JM, Fiessinger F, Poirier JE. 1980. Studies on the hydrolyzed aluminum chloride solutions: 1. Nature of aluminum species and composition of aqueous solution. J Phys Chem 84:2933–2939.
Davenport WH. 1949. Determination of aluminum in presence of iron-spectrophotometric method using ferron. Ana Chem 21:710–711.
Farmer VC. 1981. Possible roles of a mobile hydroxyaluminium orthosilicate complex (proto-imogolite) and other hydroxyaluminium and hydroxy-iron species in podzolization. Colloques Int CNRS 303:275–279.
Farmer VC, Fraser AR, Tait JM. 1979. Characterization of chemical structure of natural and synthetic aluminosilicate gels and sols by infrared spectroscopy. Geochim Cosmochim Acta 43:1417–1420.
Grim RE. 1968. Clay mineralogy. New York: McGraw-Hill. 596 p.
Hsu PH. 1989. Aluminum oxides and oxyhydroxides. In: Dixon JB, Weed SB, editors. Minerals in soil environments. Madison, WI: Soil Sci Soc Am. p 331–371.
Hsu PH. 1992. Reaction of OH-Al polymers with smectites and ventriculites. Clays Clay Miner 40:300–305.
Inoue A. 1983. Potassium fixation by clay minerals during hydrothermal treatment. Clays Clay Miner 31:81–91.
Inoue K, Pavan MA, Yoshida M. 1988. Fixation of hydroxyaluminosilicate ions (proto-imogolite) on smectite. Soil Sci Plant Nutr 34:277–285.
Inoue K, Satoh C. 1992. Electric charge and surface characteristics of hydroxyaluminosilicate- and hydroxyaluminum-vermiculite complexes. Clays Clay Miner 40:311–318.
Inoue K, Satoh C. 1993. Surface charge characteristics of hydroxyaluminosilicate- and hydroxyaluminum-vermiculite complexes. Soil Sci Soc Am J 57:547–552.
Jackson ML. 1963. Interlayering of expansible layer silicates in soils by chemical weathering. Clays Clay Miner 11:29–46.
Jackson ML. 1979. Soil chemical analysis—Advanced course, 2nd ed. Madison, WI: Jackson ML, Univ of Wisconsin. 895 p.
Kittrick JA. 1966. Forces involved in ion fixation by vermiculite. Soil Sci Soc Am Proc 30:801–803.
Kozak LM, Huang PM. 1971. Adsorption of hydroxy-Al by certain phyllosilicates and its relation to K/Ca cation exchange selectivity. Clays Clay Miner 19:95–102.
Lou G, Huang PM. 1988. Hydroxy-aluminosilicate interlayers in montmorillonite: Implications for acidic environments. Nature 335:625–627.
Lou GQJ, Huang, PM. 1994. Interlayer adsorption of hydroxy-aluminosilicate ions by montmorillonite. Soil Sci Soc Am J 58:745–750.
Mackenzie RC. 1963. Retention of exchangeable ions by montmorillonite. In: Rosenqvist Th, Graff-Petersen P, editors. Proc Int Clay Conf; 1963; Stockholm. Oxford: Pergamon Pr. p 183–193.
Manley EP, Chesworth W, Evans LJ. 1987. The solution chemistry of podzolic soils from eastern Canadian shields: A thermodynamic interpretation of mineral phases controlling soluble Al3+ and H4SiO4. J Soil Sci 38:39–51.
Matsue N, Wada K. 1988. Interlayer materials of partially interlayered vermiculite in Dystrochrepts derived from Tertiary sediments. J Soil Sci 39:155–162.
Mehra OP, Jackson ML. 1960. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonates. In: Swineford A, Plummer N, Editors. Clays Clay Miner, Proc 7th Natl Conf; 1958; Washington, DC. New York: Pergamon Pr. p 317–327.
Nagasawa K, Brown G, Newman ACD. 1974. Artificial alteration of biotite into a 14A layer silicate with hydroxyaluminum interlayers. Clays Clay Miner 22:241–252.
Page AL, Burge WD, Ganje TJ, Garber MJ. 1967. Potassium and ammonium fixation by vermiculitic soils. Soil Sci Soc Am Proc 31:337–341.
Page JB, Baver LD. 1940. Ionic size relation to fixation of cations by colloidal clay. Soil Sci Soc Am Proc 4:150–155.
Parker DR, Bertsch PM. 1992. Formation of “A113” tridecameric polycation under diverse synthesis conditions. Environ Sci Technol 26:914–919.
Rich CI. 1960. Aluminum interlayers of vermiculites. Soil Sci Soc Am Proc 24:26–32.
Rich CI. 1968. Hydroxy interlayers in expansible layer silicates. Clays Clay Miner 16:15–30.
Rich CI, Black WR. 1964. Potassium exchange as affected by cation size, pH and mineral structure. Soil Sci 97:384–390.
Rich Cl, Lutz Jr JA. 1965. Mineralogical changes associated with ammonium and potassium fixation in soil clays. Soil Sci Soc Am Proc 29:167–170.
Rich CI, Obenshain SS. 1955. Chemical and clay mineral properties of a Red-Yellow Podzolic soil derived from muscovite schist. Soil Sci Soc Am Proc 19:334–339.
Saha UK, Inoue K. 1997. Phosphate adsorption behavior of hydroxyaluminum- and hydroxyaluminosilicate-vermiculite complexes. Clay Sci 10:113–132.
Sawhney BL. 1972. Selective sorption and fixation of cations by clay minerals: A review. Clays Clay Miner 20:93–100.
Shainberg I, Kemper WD. 1966. Hydration status of adsorbed cations. Soil Sci Soc Am Proc 30:707–713.
Shoji S, Ito T, Saigusa M, Yamada I. 1985. Properties of nonallophanic Andisols from Japan. Soil Sci 140:264–277.
Shoji S, Suzuki Y, Saigusa M. 1987. Clay mineralogical and chemical properties of nonallophanic Andepts (Andisols) from Oregon, USA. Soil Sci Soc Am J 51:986–990.
Somasiri S, Huang PM. 1974. Effect of hydrolysis of aluminum on competitive adsorption of potassium and aluminum by expansible phyllosilicates. Soil Sci 117:110–116.
Stanford G. 1948. Fixation of potassium in soils under moist conditions and on drying in relation to type of clay mineral. Soil Sci Soc Am Proc 12:167–171.
Wada K, Okamura Y. 1980. Electric charge characteristics of Ando Al and buried Al horizon soils. J Soil Sci 31:307–314.
Wada S-I, Wada K. 1980. Formation, composition and structure of hydroxy-aluminosilicate ions. J Soil Sci 31:457–467.
Weaver RM, Syers JK, Jackson ML. 1968. Determination of silica in citrate-bicarbonate extracts of soils. Soil Sci Soc Am Proc 32:497–501.
Wear JI, White JL. 1951. Potassium fixation in clay minerals as related to crystal structure. Soil Sci 71:1–14.
Author information
Authors and Affiliations
Additional information
Deceased 16 August 1998.
Rights and permissions
About this article
Cite this article
Saha, U.K., Inoue, K. Hydroxy-Interlayers in Expansible Layer Silicates and Their Relation to Potassium Fixation. Clays Clay Miner. 46, 556–566 (1998). https://doi.org/10.1346/CCMN.1998.0460509
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1998.0460509