Abstract
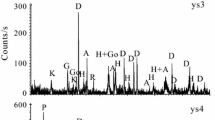
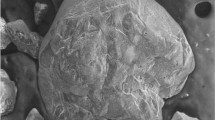
This study characterizes various chemical and mineralogical properties of goethite and jarosite from a mine drainage environment using chemical extraction techniques, X-ray diffractometry (XRD), 57Fe Mössbauer spectroscopy and scanning electron microscopy (SEM). Goethite and jarosite precipitates were collected from leachate-contaminated soils and from groundwater samples that were stored for up to 3 y. The results indicate that the soil goethites have primarily microcrystalline morphologies with moderately large mean crystallite dimensions (MCD110 ∼ 40 nm), and are superparamagnetic at room temperature and magnetically ordered at 77 K. The substitution of Al for Fe in the goethites is less than 0.03 mol/mol, and there is consequently no measured contraction in the goethite unit cell volume. The jarosite unit cell dimensions, Mössbauer parameters and chemical compositions indicate that the precipitates are primarily well-crystallized K-Na-H3O solid solutions, although the presence of poorly crystalline H3O-rich jarosite is also identified in one sample.
Similar content being viewed by others
References
Alpers CN, Nordstrom DK, Ball JW. 1989. Solubility of jarosite solid solutions precipitated from acid mine waters, Iron Mountain, California, USA. Sci Géol Bull 42:281–298.
Alpers CN, Rye RO, Nordstrom DK, White LD, King B-S. 1992. Chemical, crystallographic, and stable isotopic properties of alunite and jarosite from acid-hypersaline Australian lakes. Chem Geol 96:203–226.
Bigham, JM. 1994. Mineralogy of ochre deposits formed by sulfide oxidation. In: Jambor J, Blowes D, editors. Handbook on environmental geochemistry of sulfide mine-wastes. Mineral Assoc Can 22:103–132.
Bigham JM, Carlson L, Murad E. 1994. Schwertmannite, a new iron oxyhydroxy-sulphate from Pyhäsalmi, Finland, and other localities. Mineral Mag 58:641–648.
Bigham JM, Schwertmann U, Carlson L, Murad E. 1990. A poorly crystalline oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine water. Geochim Cosmochim Acta 54:2743–2758.
Blowes DW, Jambor JL. 1990. The pore-water chemistry and the mineralogy of the vadose zone of sulfide tailings, Waite Amulet, Quebec, Canada. Appl Geochem 5:327–346.
Brady KS, Bigham JM, Jaynes WF, Logan TJ. 1986. Influence of sulfate on Fe-oxide formation: Comparisons with a stream receiving acid mine drainage. Clays Clay Miner 34:266–274.
Brindley GW. 1980. Order-disorder in clay mineral structures. In: Brindley GW, Brown G, editors. Crystal structures of clay minerals and their X-ray identification. London: Mineral Soc. p 125–195.
Brophy GP, Scott ES, Snellgrove RA. 1962. Sulphate studies II. Solid solution between jarosite and alunite. Am Mineral 47:112–126.
Brophy GP, Sheridan FS. 1965. Sulphate studies IV: The jarosite-narojarosite-hydronium jarosite solid solution series. Am Mineral 50:1595–1607.
Campbell AS, Schwertmann U. 1984. Iron oxide mineralogy of placic horizons. J Soil Sci 35:569–582.
Carlson L, Schwertmann U. 1990. The effect of CO2 and oxidation rate on the formation of goethite versus lepido-crocite from an Fe(II) system at pH 6 and 7. Clay Miner 25:65–71.
Chapman BM, Jones DR, Jung RF. 1983. Processes controlling metal ion attenuation in acid mine drainage streams. Geochim Cosmochim Acta 47:1957–1973.
Dutrizac JE, Kaiman S. 1976. Synthesis and properties of jarosite-type compounds. Can Mineral 14:151–158.
Ficklin WH, Love AH, Papp CSE. 1991. Solid-phase variations in an aquifer as the aqueous solution changes, Globe, Arizona. In: Mallard GE, Aronson DA, editors. USGS toxic substances hydrology program, Proc Tech Meet. Water-resources investigations report 91-4034. p 475–480.
Filipek LH, Nordstrom DK, Ficklin WH. 1987. Interaction of acid mine drainage with waters and sediments of West Squaw Creek in the West Shasta Mining district, California. Environ Sci Technol 21:388–396.
Goldman DS. 1979. A reevaluation of the Mössbauer spectroscopy of calcic amphiboles. Am Mineral 64:109–118.
Herbert RB. 1994. Metal transport in groundwater contaminated by acid mine drainage. Nordic Hydrol 25:193–212.
Herbert RB. 1995a. Precipitation of Fe oxydroxides and jarosite from acidic groundwater. GFF 117:81–85.
Herbert RB. 1995b. The geochemistry of groundwater and soils contaminated by acid mine leachate: A field study from Rudolfsgruvan, Dalarna, Sweden [Ph.D. thesis]. Uppsala, Sweden: Institution of Earth Sciences, Uppsala Univ. 133 p.
Herbert RB. 1996. Metal retention by iron oxide precipitation from acid ground water in Dalarna, Sweden. Applied Geochem 11:229–236.
Jambor JL. 1994. Mineralogy of sulfide-rich tailings and their oxidation products. In: Jambor J, Blowes D, editors. Handbook on environmental geochemistry of sulfide mine-wastes. Mineral Assoc Can 22:59–102.
JCPDS, Joint Committee on Powder Diffraction Standards. 1972. Selected powder diffraction data for minerals. Publication DBM-1-23. Swarthmore, PA: JCPDS.
Johnson CA. 1986. The regulation of trace element concentrations in river and estuarine waters contaminated with acid mine drainage: The adsorption of Cu and Zn on amorphous Fe oxyhydroxides. Geochim Cosmochim Acta 50:2433–2438.
Karlsson S, Allard B, Håkansson K. 1988. Chemical characterization of stream-bed sediments receiving high loadings of acid mine effluents. Chem Geol 67:1–15.
Klug HP, Alexander LE. 1974. X-ray diffraction procedures for polycrystalline and amorphous materials. New York: J Wiley. 966 p.
Langmuir D, Whittemore DO. 1971. Variations in the stability of precipitated ferric oxyhydroxides. In: Gould RF, editor. Nonequilibrium systems in natural water chemistry. Adv Chem Ser 106:209–234.
Leclerc A. 1980. Room temperature Mössbauer analysis of jarosite-type compounds. Phys Chem Miner 6:327–334.
Murad E. 1982. The characterization of goethite by Mössbauer spectroscopy. Am Mineral 67:1007–1011.
Murad E. 1988. Properties and behavior of iron oxides as determined by Mössbauer spectroscopy. In: Stucki JW, Goodman BA, Schwertmann U, editors. Iron in soils and clay minerals. Dordrecht: D. Reidel. p 309–350.
Murad E, Bigham JM, Bowen LH, Schwertmann U. 1990. Magnetic properties of iron oxides produced by bacterial oxidation of Fe2+ under acid conditions. Hyperfine Interact 58:2373–2376.
Murad E, Schwertmann U. 1980. The Mössbauer spectrum of ferrihydrite and its relations to those of other iron oxides. Am Mineral 65:1044–1049.
Nordstrom DK. 1982. Aqueous pyrite oxidation and the consequent formation of secondary iron minerals. In: Hossner LR, editor. Acid sulfate weathering. SSSA publication 10. Madison: Soil Sci Soc Am. p 95–108.
Postma D. 1993. The reactivity of iron oxides in sediments: A kinetic approach. Geochim Cosmochim Acta 57:5027–5034.
Ripmeester JA, Ratcliffe CI, Dutrizac JE, Jambor JL. 1986. Hydronium ion in the alunite-jarosite group. Can Mineral 24:435–447.
Schulze DG. 1981. Identification of soil iron oxide minerals by differential X-ray diffraction. Soil Sci Soc Am J 45:437–440.
Schulze DG. 1984. The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays Clay Miner 32:36–44.
Schulze DG, Schwertmann U. 1984. The influence of aluminum on iron oxides. X. Properties of Al-substituted goethites. Clay Miner 19:521–539.
Schwertmann U. 1964. Differenzierung der Eisenoxides des Bodens durch photochemische Extraktion mit saurer Ammoniumoxalat-Lösung. Z Pflanzenernähr Bodenkd 105:194–202.
Schwertmann U. 1973. Use of oxalate for Fe extraction from soils. Can J Soil Sci 53:244–246.
Schwertmann U. 1985. The effect of pedogenic environments on iron oxide minerals. Adv Soil Sci 1:172–200.
Schwertmann U, Cambier P, Murad E. 1985. Properties of goethites of varying crystallinity. Clays Clay Miner 33:369–378.
Schwertmann U, Carlson L. 1994. Aluminum influence on iron oxides: XVII. Unit-cell parameters and aluminum substitution on natural goethites. Soil Sci Soc Am J 58:256–261.
Schwertmann U, Carlson L, Murad E. 1987. Properties of iron oxides in two Finnish lakes in relation to the environment of their formation. Clays Clay Miner 35:297–304.
Schwertmann U, Cornell RM. 1991. Iron oxides in the laboratory: Preparation and characterization. Weinheim: VCH Verlagsgesellschaft mbH. 137 p.
Schwertmann U, Taylor RM. 1989. Iron oxides. In: Dixon JB, Weed SB, editors. Minerals in soil environments, 2nd ed. Madison, WI: Soil Sci Soc Am. p 379–438.
Stollenwerk K. 1994. Geochemical interactions between constituents in acidic groundwater and alluvium in an aquifer near Globe, Arizona. Applied Geochem 9:353–369.
Ribet I, Ptacek CJ, Blowes DW, Jambor JL. 1995. The potential for metal release by reductive dissolution of weathered mine tailings. J Contam Hydrol 17:239–273.
WaveMetrics. 1994. Igor Pro user’s manual 2.00. Lake Oswego, OR: WaveMetrics Inc. 1080 p.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herbert, R.B. Properties of Goethite and Jarosite Precipitated from Acidic Groundwater, Dalarna, Sweden. Clays Clay Miner. 45, 261–273 (1997). https://doi.org/10.1346/CCMN.1997.0450214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1997.0450214