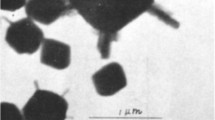
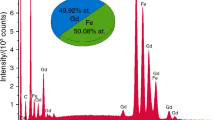
Abstract
This work investigates unit cell dimensions, crystal size and specific surface area of aluminous goethite that was progressively dehydroxylated to form hematite. Goethite synthesized from the ferrous system altered to hematite with DTGA maximum increasing from 236° to 273°C for 0 to 30.1 mole % Al-substitution. Unit cell dimensions of goethite and hematite decreased as Al-substitution increased and increased as excess OH increased. The crystallographically equivalent a axis of goethite and c axis of hematite were more sensitive than other axes to the presence of excess structural OH associated with Al-substitution. Specific surface area increased from 147 to 288 m2/g for goethite and from 171 to 230 m2/g for hematite as Al-substitution increased. An increase in specific surface area on heating goethite at temperatures between 200° and 240°C is related to a decrease in the size of coherently diffracting domains of goethite crystals and to the development of pore and structural defects associated with the formation of hematite. The decrease in specific surface area for heating temperatures above 240°C is attributed to the growth of hematite crystals by diffusion.
Similar content being viewed by others
References
Anand, R. R., and R. J. Gilkes. 1984. Weathering of hornblende, plagioclase and chlorite in Meta-Dolerite Australia. Geoderma 34: 261–280.
Bemal, J. D., D. R. Dasgupta, and A. L. Mackay. 1959. The oxides and hydroxides of iron and their structural interrelationships. Clay Miner. Bulletin 4: 15–30.
Brown, G. 1980. Crystal Structure of Clay Minerals and their X-ray Identification. G. W. Brindley and G. Brown, eds. London: Mineralogical Society, pp. 361–410.
DeGrave, E., L. H. Bowen, and S. B. Weed. 1982. Mössbauer study of aluminum substituted hematites. J. Magn. & Magn. Mat. 72: 129–140.
Fazey, P. G., B. H. O’Connor, and L. C. Hammond. 1991. X-Ray powder diffraction rietveld characterization of synthetic aluminum-substituted goethite. Clays & Clay Miner. 39: 248–253.
Fey, M. B., and J. B. Dixon. 1981. Synthesis and properties of poorly crystalline hydrated aluminous goethites. Clays & Clay Miner. 29: 91–100.
Francombe, M. H., and H. P. Rooksby. 1959. Structure transformations effected by the dehydration of diaspore, goethite and delta ferric oxide. Clay Miner. Bulletin 21: 1–14.
Goodman, B. A., and D. G. Lewis. 1981. Mössbauer spectra of aluminous goethite (α-FeOOH). J. Soil Sci. 32: 351–363.
Goss, C. J. 1987. The kinetics and reaction mechanism of the goethite to hematite transformation. Miner. Mag. 51: 437–451.
Gregg, S. J., and K. J. Hill. 1953. The production of active solids by thermal decomposition. Part II. Ferric oxide. J. Chem. Soc. IV: 3945–3951.
Jónás, K., and K. Solymár. 1970. Preparation, X-ray, derivatographic and infrared study of aluminium-substituted goethites. Acta Chim. Acad. Sci. Hung. 66: 383–394.
Lewis, D. G., and U. Schwertmann. 1979. The influence of Al on iron oxides. III. Preparation of Al-goethite in M KOH. Clay Miner. 14: 115–126.
Lewis, D. G., and U. Schwertmann. 1980. The effect of [OH] on the goethite produced from ferrihydrite under alkaline conditions. J. Colloid Interface Sci. 78: 543–553.
Lim-Nunez, R. S. L. 1985. Synthesis and acid dissolution of metal-substituted goethites and hematites: MSc. thesis. Department of Soil and Plant Nutrition, U.W.A., Nedlands, 6009, pp. 104–121.
Mackenzie, R. C., and G. Berggren. 1970. Oxides and hydroxides of higher valence elements. In Differential Thermal Analysis 1. R. C. Mackenzie, ed. New York: Academic Press, 271–302.
McKeague, J. A., and J. H. Day. 1966. Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 46: 13–22.
Naono, H., and R. Fujiwara. 1980. Micropore formation due to thermal decomposition of acicular microcrystals of α-FeOOH. J. Colloid Interface Sci. 73: 406–415.
Novak, G. A., and A. A. Colville. 1989. A practical interactive least-squares cell-parameter program using an electronic spreadsheet and a personal computer. Amer. Miner. 74: 488–490.
Okamoto, G., R. Furuichi, and N. Sato. 1967. Chemical reactivity and electrical conductivity of hydrous ferric oxide. Electrochim. Acta 12: 1287–1299.
Permet, G., and R. Lafont. 1972a. Sur le paramétres cristallographiques des hématites aluminineuses. C. R. Acad. Sci. 275: 1021–1024.
Perinet, G., and R. Lafont. 1972b. Sur la présence d’hématite aluminineuses désordonée dans des bauxites du Var. C. R. Acad. Sci. 274: 272–274.
Rendon, J. L., J. Cornejo, P. Dearambarri, and C. J. Serna. 1983. Pore structure of thermally treated goethite (α-FeOOH). J. Colloid Interface Sci. 92: 508–516.
Schulze, D. G. 1982. The identification of iron oxides by differential X-ray diffraction and the influence of aluminum substitution on the structure of goethite: Ph.D. thesis. Lehrstuhl für Bodenkunde der Technischen Universität München, Weihenstephan.
Schulze, D. G. 1984. The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clay & Clay Miner. 32: 36–44.
Schulze, D. G., and U. Schwertmann. 1984. The influence of aluminium on iron oxides: X. Properties of Al-substituted goethites. Clay Miner. 19: 521–539.
Schulze, D. G., and U. Schwertmann. 1987. The influence of aluminium on iron oxides: XIII. Properties of goethites synthesised in 0.3 M KOH at 25°C. Clay Miner. 22: 83–92.
Schwertmann, U., P. Cambier, and E. Murad. 1985. Properties of goethites of varying crystallinity. Clays & Clay Miner. 33: 369–378.
Schwertmann, U., R. W. Fitzpatrick, R. M. Taylor, and D. G. Lewis. 1979. The influence of aluminium on iron oxides. Part II. Preparation and properties of Al-substituted hematites. Clays & Clay Miner. 27: 105–112.
Singh, B., and R. J. Gilkes. 1992. XPAS: An interactive computer program for analysis of X-ray powder diffraction patterns. Powder Diffraction 7: 6–10.
Stanjek, H., and U. Schwertmann. 1992. The influence of aluminium on iron oxides. Part XVI: Hydroxyl and aluminium substitution in synthetic hematites. Clays & Clay Miner. 40: 347–354.
Taylor, M. R., and U. Schwertmann. 1978. The influence of aluminium on iron oxides. Part I. The influence of Al on Fe oxide formation from the Fe (II) system. Clays & Clay Miner. 26: 373–383.
Thiel, R. 1963. Zum system α-FeOOH-α-AlOOH. Z. Anorg. Allg. Chem. 326: 70–78.
Watari, F., J. van Landuyt, P. Delavignette, and S. Amelinckx. 1979. Electron microscopic study of dehydration transformations I. Twin formation and mosaic structure in hematite derived from goethite. J. Solid State Chem. 29:137–150.
Watari, F., P. Delavignette, J. van Landuyt, and S. Amelinckx. 1983. Electron microscopic study of dehydration transformations III. High resolution observation of the reaction process FeOOH → Fe2O3. J. Solid State Chem. 48: 49–64.
Wells, M. A., R. J. Gilkes, and R. R. Anand. 1989. The formation of corundum and aluminous hematite by the thermal dehydroxylation of aluminous goethite. Clay Miner. 24: 513–530.
Wolska, E., 1981. The structure of hydrohematite. Z. Kristallographie 154: 69–75.
Wolska, E., and U. Schwertmann. 1989. Nonstoichiometric structures during dehydroxylation of goethite. Z. Kristallographie 189: 223–237.
Wolska, E., and W. Szajda. 1985. Structural and spectroscopic characteristics of synthetic hydrohematite. J. Mater. Sci. 20:4407–4412.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ruan, H.D., Gilkes, R.J. Dehydroxylation of Aluminous Goethite: Unit Cell Dimensions, Crystal Size and Surface Area. Clays Clay Miner. 43, 196–211 (1995). https://doi.org/10.1346/CCMN.1995.0430207
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1995.0430207