Abstract
The amount of Mn2+ adsorbed or removed from solution by birnessite is several times greater than its reported cation exchange capacity. Extractability of the sorbed Mn2+ decreases with aging. It is uncertain whether the sorbed Mn2+ is oxidized on the surface or incorporated into the structure of birnessite. Using X-ray powder diffractometry and transmission electron microscopy, a study was conducted to examine the mineralogical alteration of birnessite after treatment with various concentrations of MnSO4 and solution pH.
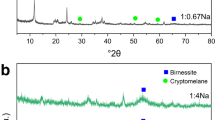
The sorbed Mn2+ was not directly oxidized and remained on the birnessite surface. The sorption of Mn2+ was followed by alteration of birnessite with the formation of new Mn minerals. The specific Mn minerals formed were governed by the pH of the reaction, and the rate of the transformation was determined by Mn2+ concentration and pH. Nsutite and ramsdellite were identified at pH 2.4, crypto-melane at pH 4, groutite at pH 6, and manganite at pH 8. Other Mn minerals formed at these and other pH levels could not be identified. As the concentration of Mn in the solution decreased, the time required to form new minerals from the birnessite increased. The newly formed phases were the result of structural conversion since dissolution of birnessite and reprecipitation of new phases were not observed.
Similar content being viewed by others
References
Brown, F. H., Pabst, A., and Sawyer, D. L. (1971) Birnessite on colemanite at Boron, California: Amer. Mineral. 56, 1057–1064.
Buser, W. P. and Feitknecht, W. (1954) Beitrag zur Kenntnis der Mangan(II)-manganite und des 3-MnO2: Helv. Chim. Acta 37, 2322–2333.
Davis, J. A. and D. B. Kent. (1990) Surface complexation modelling in aqueous geochemistry: in Mineral-water Interface Geochemistry: Reviews in Mineralogy, 23, M. F. Hochella Jr. and A. F. White, eds., 177–260.
Feitknecht, W., Oswald, H. R., and Feitknecht-Steimann, V. (1960) Über die topochemische einphasige reduktion von 7-MnO2. Helv. Chim. Acta. 48, 1947–1950.
Fendorf, S. E., Sparks, D. L., Franz, J. A., and Camaioni, D. M. (1993) Electron paramagnetic resonance stopped-flow kinetic study of manganese(II) sorption-desorption on bir-nessite: Soil Sci. Soc. Am. J. 57, 57–62.
Frondel, O., Mervin, O. B., and Ito, J. (1960) New data on birnessite and hollandite: Amer. Mineral. 45, 871–875.
Gattow, G. and Glemser, O. (1961) Darstellung und Eigen-schaften von Braunstein. Part II: Die γ-and η-Gruppe der Brausteine: Z. Anorg. Allg. Chem. 309, 20–36.
Glemser, O., Gattow, G., and Heisiek, H. (1961) Darstellung und Eigenschaften von Braunstein. Part I: Die 5-Gruppe der Brausteine: Z. Anorg. Allg. Chem. 309, 1–19.
Golden, D. C., Dixon, J. B., and Cheng, C. C. (1986) Ion exchange, thermal transformations, and oxidizing proper-ties of birnessite: Clays & Clay Minerals 34, 511–520.
Healy, T. W., Herring, A. P., and Fuerstenau, D. W. (1966) The effect of crystal structure on the surface properties of a series of manganese dioxides: J. Colloid Interface Sci. 21, 435–444.
Hem, J. D. (1963) Chemical equilibria and rates of man-ganese of oxidation: US. Geol. Surv. Water Supply Paper 1667-A.
JCPDS—International Centre for Diffraction Data. (1992) Feint-Marquart’s μPDSM micropowder diffraction search/ match. Release 4.30. Pennsylvania, USA.
Jones, L. H. P. and Milne, A. A. (1956) Birnessite, a new manganese oxide mineral from Aberdeenshire, Scotland: Mineral Mag. 31, 283–288.
Koljonen, T., Lahermo, P., and Garlson, L. (1976) Origin, mineralogy and geochemistry of manganese rocks and fer-ruginous precipitates found in sand graveldeposits in Finland: Bull. Geol. Soc. Finland 48, 111–135.
Krauskopf, K. B. (1972) Geochemistry of micronutrients: in Micronutrients in Agriculture, J. J. Mortvedt, F. R. Cox, L. M. Shuman, and R. M. Walsh, eds., Soil Science Society of America, Madison, Wisconsin, 7–36.
McKenzie, R. M. (1971) The synthesis of birnessite, cryp-tomelane and some other oxides and hydroxides of manganese: Mineral Mag. 38, 493–502.
McKenzie, R. M. (1980a). The adsorption of lead and other heavy metals on oxides of manganese and iron. Aust. J. Soil Res. 18, 61–73.
McKenzie, R. M. (1980b) The manganese oxides in soils: in Geology and Geochemistry of Manganese, Vol. I, I. M. Varentsov and Gy Grasselly, eds., Hungarian Academy of Science, Budapest, 259–269.
McKenzie, R. M. (1981) The surface charge on manganese dioxide: Aust. J. Soil. Sci. Res. 19, 41–50.
McKenzie, R. M. (1989) The manganese oxides and hydroxides: in Minerals in Soil Environments, J. B. Dixon and S. B. Weed, eds., Soil Science Society of America, Madison, Wisconsin, 439–465.
Morgan, J. J. and Stumm, W. (1964) The role of multivalent metal oxides in limnological transformations as exemplified by iron and manganese: Adv. Water Pollut. Res., Proc. Int. Conf. 2nd. (Tokyo), Pergamon Press, Oxford. 103–118.
Murray, J. W. and Dillard, J. G. (1979) The oxidation of cobalt(II) adsorbed on manganese oxide: Geochim. Cosmochim. Acta. 43, 781–787.
Pankow, J. F. and Morgan, J. J. (1981) Kinetics for the aquatic environment: Environmental Sci. and Tech, 15, 1306–1313.
Sung, W. and Morgan, J. J. (1981) Oxidative removal of Mn(II) from solution catalysed by the γ-FeOOH (lepidocrocite) surface: Geochim. Cosmochim. Acta 45, 2377–2383.
Tu, S. (1993) Effects of KCl on solubility and bioavailability of Mn in soil and some reactions of birnessite in the presence of some Mn compounds. Ph.D. Dissertation, University of Manitoba, 112 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tu, S., Racz, G.J. & Goh, T.B. Transformations of Synthetic Birnessite as Affected by pH and Manganese Concentration. Clays Clay Miner. 42, 321–330 (1994). https://doi.org/10.1346/CCMN.1994.0420310
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1994.0420310