Abstract
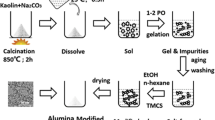
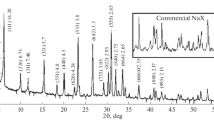
Kaolinite was hydrothermally synthesized from two kinds of silica-alumina gels to examine the effect of the structure of the starting material. Two kinds of gels were prepared by precipitation at different pH conditions (pH = 9.6 and 4.2) from solutions containing water glass and aluminum sulfate. Na ions in the gels were removed with a resin before the hydrothermal treatment, but a slight amount of sulfate ions was still present in the gels. The nuclear magnetic resonance spectra of the starting gels suggested that the gel prepared at pH 9.6 consists of networks with alternating SiO4- and A1O4-tetrahedra (partially AlO6-octahedra), whereas the gel prepared at pH 4.2 consists of a sheet structure related to allophane. After the hydrothermal treatment at 220°C for 9 days, kaolinite particles with spherical shape were obtained from the former gel, and platy kaolinite was crystallized from the latter one. The difference in morphology of synthetic kaolinite was attributable to the structures of the starting gels, and the pH values in the hydrothermal reactions were not very significant to the morphology.
Similar content being viewed by others
References
Barron, P. F., Wilson, M. A., Campbell, A. S., and Frost, R. L. (1982) Detection of imogolite in soils using solid state 29Si NMR: Nature 299, 616–618.
Barron, P. F., Frost, R. L., Skjemstad, J. O., and Koppi, A. J. (1983) Detection of two silicon environments in kaolins by solid-state 29Si NMR: Nature 302, 49–50.
Cloos, P., Léonard, A. J., Moreau, J. P., Herbillon, A., and Fripiat, J. J. (1969) Structural organization in amorphous silico-aluminas: Clays & Clay Minerals 17, 279–287.
De Kimpe, C., Gastuche, M. C., and Brindley, G. W. (1961) Ionic coordination in alumino-silicic gels in relation to clay mineral formation: Amer. Mineral. 46, 1370–1381.
De Kimpe, C. and Gastuche, M. C. (1964) Low-temperature syntheses of kaolin minerals: Amer. Mineral. 49, 1–16.
Ewell, R. H. and Insley, H. (1935) Hydrothermal synthesis of kaolinite, dickite, beidellite, and nontronite: J. Res. Nat. Bur. Stand. 15, 173–186.
Fripiat, J. J., Léonard, A., and Uytterhoeven, J. B. (1965) Structure and properties of amorphous silicoaluminas. II. Lewis and brensted acid sites: J. Phys. Chem. 69, 3274–3279.
Holdridge, D. A. and Vaughan, F. (1957) The kaolin min-erals (Kandites): in The Differential Thermal Investigation of Clays, R. C. Mackenzie, ed., Mineralogical Society, London, 98–139.
Léonard, A., Suzuki, Sho., Fripiat, J. J., and De Kimpe, C. (1964) Structure and properties of amorphous silicoaluminas. I. Structure from X-ray fluorescence spectroscopy and infrared spectroscopy: J. Phys. Chem. 68, 2608–2617.
Lippmaa, E., Mägi, M., Samoson, A., Engelhardt, G., and Grimmer, A.-R. (1980) Structural studies of silicates by solid-state high-resolution 29Si NMR: J. Am. Chem. Soc. 102, 4889–4893.
Lippmaa, E., Mägi, M., Samoson, A., Tarmak, M., and Engelhardt, G. (1981) Investigation of the structure of zeolites by solid-state high-resolution 29Si NMR spectroscopy: J. Am. Chem. Soc. 103, 4992–4996.
MacKenzie, K. J. D., Bowden, M. E., and Meinhold, R. H. (1991) The structure and thermal transformations of allophanes studied by 29Si and 27Al high resolution solid-state NMR: Clays & Clay Minerals 39, 337–346.
Miyawaki, R., Tomura, S., Shibasaki, Y., and Samejima, S. (1989) Appropriate pH for hydrothermal synthesis of kaolinite from amorphous mixture of alumina and silica: Reports of the Government Industrial Research Institute, Nagoya 38, 330–335 (in Japanese with English abstract).
Miyawaki, R., Tomura, S., Samejima, S., Okazaki, M., Mi-zuta, H., Maruyama, S., and Shibasaki, Y. (1991) Effects of solution chemistry on the hydrothermal synthesis of kaolinite: Clays & Clay Minerals, 39, 498–508.
Pourbaix, M., ed. (1991) Atlas of Electrochemical in Aqueous Solutions: Pergamon Press, New York, 168–176, 458–463 pp.
Roy, R. and Osborn, E. F. (1954) The system Al2O3-SiO2-H2O: Amer. Mineral. 39, 853–885.
Thompson, J. G., Philippa, J. R. U., Whittaker, A. K., and Mackinnon, I. D. R. (1992) Structural characterization of kaolinite:NaCl intercalate and its derivatives: Clays & Clay Minerals, 40, 369–380.
Tomura, S., Shibasaki, Y., Mizuta, H., and Kitamura, M. (1983) Spherical kaolinite: Synthesis and mineralogical properties: Clays & Clay Minerals 31, 413–421.
Tomura, S., Shibasaki, Y., Mizuta, H., and Kitamura, M. (1985) Growth conditions and genesis of spherical and platy kaolinite: Clays & Clay Minerals 33, 200–206.
Tsuzuki, Y. (1976) Solubility diagrams for explaining zone sequences in bauxite, kaolin and pyrophyllite-diaspore de-posits: Clays & Clay Minerals 24, 297–302.
Van der Marel, H. W. and Beutelspacher, H., eds. (1976) Atlas of Infrared Spectroscopy of Clay Minerals and Their Admixtures: Elsevier, New York, 65–93.
Watanabe, T. and Shimizu, H. (1986) High resolution solid state NMR and its application to clay minerals: J. Mineral Soc. Jpn. 17, 123–136 (special issue, in Japanese with English abstract).
Wilson, M. A., McCarthy, S. A., and Fredericks, P. M. (1986) Structure of poorly-ordered aluminosilicates: Clay Miner. 21, 879–897.
Wilson, M. A., Wada, K., Wada, S. I., and Kakuto, Y. (1988) Thermal transformations of synthetic allophane and imogolite as revealed by nuclear magnetic resonance: Clay Miner. 23, 175–190.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satokawa, S., Osaki, Y., Samejima, S. et al. Effects of the Structure of Silica-Alumina Gel on the Hydrothermal Synthesis of Kaolinite. Clays Clay Miner. 42, 288–297 (1994). https://doi.org/10.1346/CCMN.1994.0420307
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1994.0420307